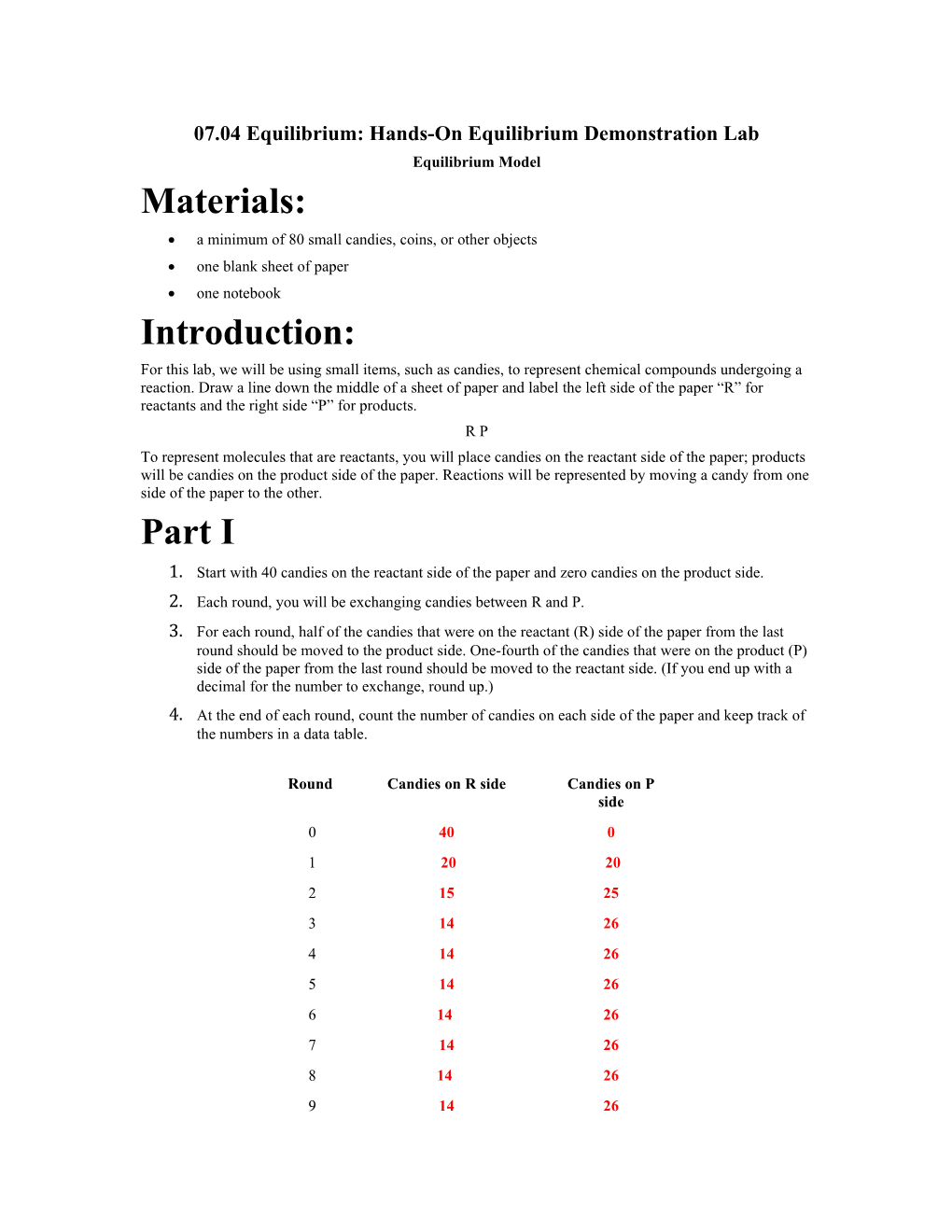
07.04 Equilibrium: Hands-On Equilibrium Demonstration Lab Equilibrium Model Materials: a minimum of 80 small candies, coins, or other objects one blank sheet of paper one notebook Introduction: For this lab, we will be using small items, such as candies, to represent chemical compounds undergoing a reaction. Draw a line down the middle of a sheet of paper and label the left side of the paper “R” for reactants and the right side “P” for products. R P To represent molecules that are reactants, you will place candies on the reactant side of the paper; products will be candies on the product side of the paper. Reactions will be represented by moving a candy from one side of the paper to the other. Part I 1. Start with 40 candies on the reactant side of the paper and zero candies on the product side. 2. Each round, you will be exchanging candies between R and P. 3. For each round, half of the candies that were on the reactant (R) side of the paper from the last round should be moved to the product side. One-fourth of the candies that were on the product (P) side of the paper from the last round should be moved to the reactant side. (If you end up with a decimal for the number to exchange, round up.) 4. At the end of each round, count the number of candies on each side of the paper and keep track of the numbers in a data table.
Round Candies on R side Candies on P side 0 40 0 1 20 20 2 15 25 3 14 26 4 14 26 5 14 26 6 14 26 7 14 26 8 14 26 9 14 26 10 14 26
Ratio = 0.7
1. Follow the instructions in Step 3 for a total of 10 rounds. 2. At the end of 10 rounds, calculate the ratio (ratio = P/R) of products to reactants. Part II Part II will be the same procedure as Part I, except you will be starting with different initial amounts of products and reactants. 1. Choose a number of candies to put on the reactant side and put the rest on the products side. 2. For each round, half of the candies that were on the reactant (R) side of the paper from the last round should be moved to the product side. One-fourth of the candies that were on the product (P) side of the paper from the last round should be moved to the reactant side. (If you end up with a decimal for the number to exchange, round up.) 3. At the end of each round, count the number of candies on each side of the paper and keep track of the numbers in a data table. 4. Follow the instructions in Step 2 for a total of 10 rounds. 5. At the end of 10 rounds, calculate the ratio (ratio = P/R ) of products to reactants.
Round Candies on R side Candies on P side 0 20 20 1 5 10 2 2.5 2.5 3 0.6 1.3 4 0.3 0.3 5 0.8 0.15 6 0.04 0.4 7 0.1 0.02 8 0.005 0.05 9 0.03 0.003 10 0.0008 0.01 Ratio = 1.2
Part III Part III follows a similar procedure as Part I and Part II, except you will be adding additional candies to the activity. 1. Start with 40 candies on the reactant side of the paper. 2. For each round, half of the candies that were on the reactant (R) side of the paper from the last round should be moved to the product side. One-fourth of the candies that were on the product (P) side of the paper from the last round should be moved to the reactant side. (If you end up with a decimal for the number to exchange, round up.) 3. At the end of each round, count the number of candies on each side of the paper and keep track of the numbers in a data table. 4. After five rounds, add 40 more candies to the reactant side of the paper. Continue following the instructions in Step 2 with this new amount of candy (total of 80 pieces of candy) for an additional 10 rounds. 5. At the end of 15 total rounds, calculate the ratio (ratio = P/R) of products to reactants.
Round Candies on R side Candies on P side 0 1 2 3 4 5
6 7 8 9 10
11
12
13
14
15
Mr. Clark,
Sorry it took me so long to submit this assignment its just that it didn’t seem like I did it right but I think I did it right, its kind of weird how the ratio didn’t change I though it was going to change.