FOR ONLINE PUBLICATION ONLY
Appendix A: List of Studies Used in the Meta-analyses
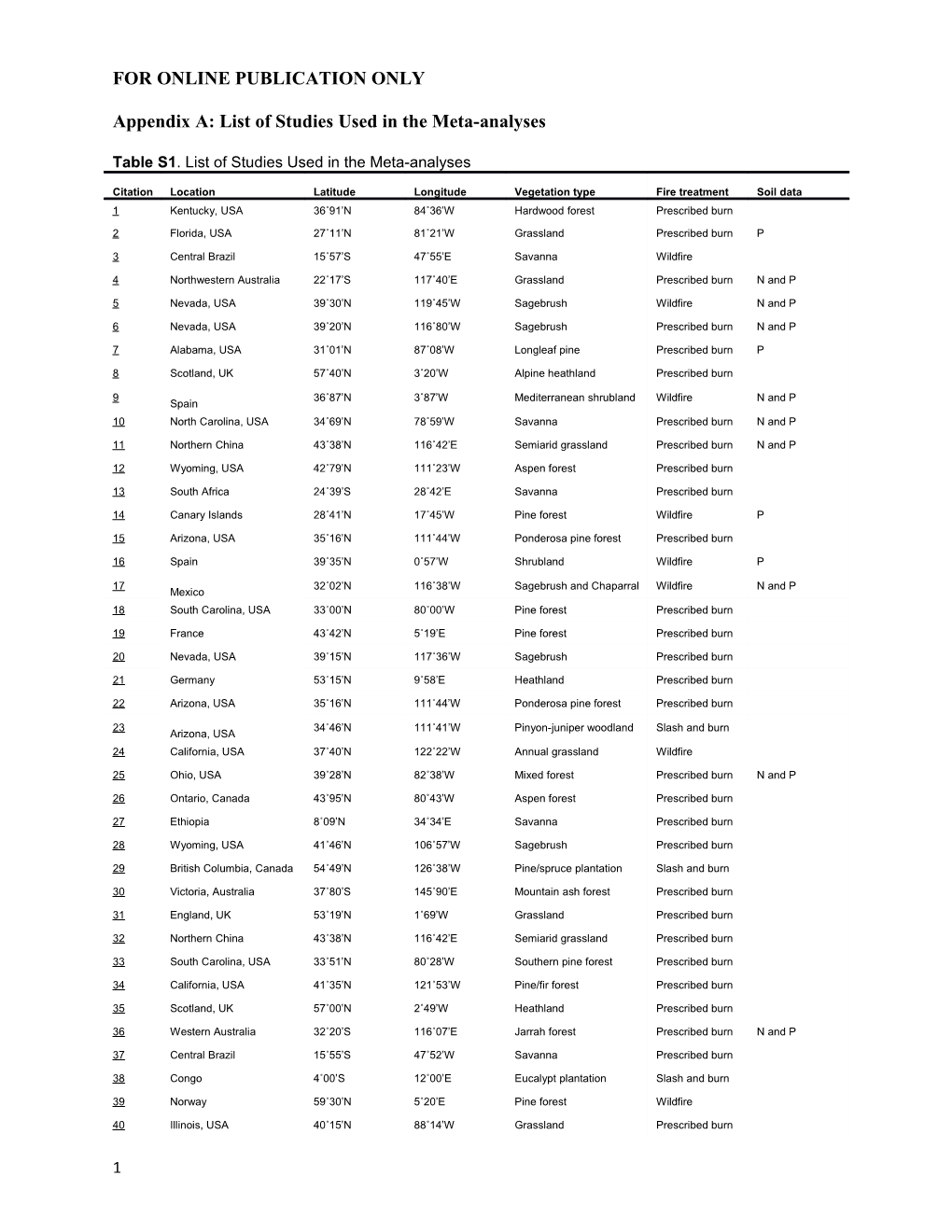
Table S1. List of Studies Used in the Meta-analyses
Citation Location Latitude Longitude Vegetation type Fire treatment Soil data 1 Kentucky, USA 36˚91’N 84˚36’W Hardwood forest Prescribed burn
2 Florida, USA 27˚11’N 81˚21’W Grassland Prescribed burn P
3 Central Brazil 15˚57’S 47˚55’E Savanna Wildfire
4 Northwestern Australia 22˚17’S 117˚40’E Grassland Prescribed burn N and P
5 Nevada, USA 39˚30’N 119˚45’W Sagebrush Wildfire N and P
6 Nevada, USA 39˚20’N 116˚80’W Sagebrush Prescribed burn N and P
7 Alabama, USA 31˚01’N 87˚08’W Longleaf pine Prescribed burn P
8 Scotland, UK 57˚40’N 3˚20’W Alpine heathland Prescribed burn
9 36˚87’N 3˚87’W Mediterranean shrubland Wildfire N and P Spain 10 North Carolina, USA 34˚69’N 78˚59’W Savanna Prescribed burn N and P
11 Northern China 43˚38’N 116˚42’E Semiarid grassland Prescribed burn N and P
12 Wyoming, USA 42˚79’N 111˚23’W Aspen forest Prescribed burn
13 South Africa 24˚39’S 28˚42’E Savanna Prescribed burn
14 Canary Islands 28˚41’N 17˚45’W Pine forest Wildfire P
15 Arizona, USA 35˚16’N 111˚44’W Ponderosa pine forest Prescribed burn
16 Spain 39˚35’N 0˚57’W Shrubland Wildfire P
17 32˚02’N 116˚38’W Sagebrush and Chaparral Wildfire N and P Mexico 18 South Carolina, USA 33˚00’N 80˚00’W Pine forest Prescribed burn
19 France 43˚42’N 5˚19’E Pine forest Prescribed burn
20 Nevada, USA 39˚15’N 117˚36’W Sagebrush Prescribed burn
21 Germany 53˚15’N 9˚58’E Heathland Prescribed burn
22 Arizona, USA 35˚16’N 111˚44’W Ponderosa pine forest Prescribed burn
23 34˚46’N 111˚41’W Pinyon-juniper woodland Slash and burn Arizona, USA 24 California, USA 37˚40’N 122˚22’W Annual grassland Wildfire
25 Ohio, USA 39˚28’N 82˚38’W Mixed forest Prescribed burn N and P
26 Ontario, Canada 43˚95’N 80˚43’W Aspen forest Prescribed burn
27 Ethiopia 8˚09’N 34˚34’E Savanna Prescribed burn
28 Wyoming, USA 41˚46’N 106˚57’W Sagebrush Prescribed burn
29 British Columbia, Canada 54˚49’N 126˚38’W Pine/spruce plantation Slash and burn
30 Victoria, Australia 37˚80’S 145˚90’E Mountain ash forest Prescribed burn
31 England, UK 53˚19’N 1˚69’W Grassland Prescribed burn
32 Northern China 43˚38’N 116˚42’E Semiarid grassland Prescribed burn
33 South Carolina, USA 33˚51’N 80˚28’W Southern pine forest Prescribed burn
34 California, USA 41˚35’N 121˚53’W Pine/fir forest Prescribed burn
35 Scotland, UK 57˚00’N 2˚49’W Heathland Prescribed burn
36 Western Australia 32˚20’S 116˚07’E Jarrah forest Prescribed burn N and P
37 Central Brazil 15˚55’S 47˚52’W Savanna Prescribed burn
38 Congo 4˚00’S 12˚00’E Eucalypt plantation Slash and burn
39 Norway 59˚30’N 5˚20’E Pine forest Wildfire
40 Illinois, USA 40˚15’N 88˚14’W Grassland Prescribed burn
1 41 Cameroon 6˚02’N 10˚17’E Maize Slash and burn P
42 New Zealand 45˚37’S 169˚20’E Grassland Prescribed burn
43 Arizona, USA 35˚29’N 111˚63’W Ponderosa pine forest Wildfire
44 North Carolina, USA 36˚25’N 78˚30’W Loblolly pine plantation Slash and burn
45 Nevada, USA 39˚15’N 117˚35’W Sagebrush Prescribed burn N and P
46 Wisconsin, USA 44˚54’N 89˚56’W Hardwood forest Prescribed burn
47 New Zealand 45˚36’S 169˚16’E Grassland Prescribed burn N and P
48 California, USA 36˚56’N 118˚52’W Chaparral Wildfire N and P
49 Florida, USA 27˚11’N 81˚21’W Shrubland Prescribed burn N and P
50 India 24˚36’N 83˚30’E Savanna Prescribed burn
51 Kansas, USA 39˚22’N 96˚62’W Grassland Prescribed burn
52 Venezuela 1˚56’N 67˚03’W Tropical forest Slash and burn
53 Tanzania 4˚00’S 37˚00’E Savanna Prescribed burn
54 Germany 53˚15’N 9˚58’E Heathland Prescribed burn Last column indicates in which studies measurements of soil available N and/or P were included
References
1 Adams, A. S. & Rieske, L. K. Prescribed fire affects white oak seedling phytochemistry: Implications for insect herbivory. For. Ecol. Manag. 176, 37-47 (2003). 2 Anderson, R. C. & Menges, E. S. Effects of fire on sandhill herbs: Nutrients, mycorrhizae, and biomass allocation. Am. J. Bot. 84, 938-948 (1997). 3 Batmanian, G. J. & Haridasan, M. Primary production and accumulation of nutrients by the ground layer community of cerrado vegetation of central Brazil. Plant Soil 88, 437- 440 (1985). 4 Bennett, L. T., Judd, T. S. & Adams, M. A. Growth and nutrient content of perennial grasslands following burning in semi-arid, sub-tropical Australia. Plant Ecol. 164, 185- 199 (2002). 5 Blank, R. R., Allen, F. & Young, J. A. Growth and elemental content of several sagebrush-steppe species in unburned and post-wildfire soil and plant effects on soil attributes. Plant Soil 164, 35-41 (1994). 6 Blank, R. R., Chambers, J. C. & Zamudio, D. Restoring riparian corridors with fire: Effects on soil and vegetation. J. Range Manag. 56, 388-396 (2003). 7 Boyer, W. D. & Miller, J. H. Effect of burning and brush treatments on nutrient and soil physical-properties in young longleaf pine stands. For. Ecol. Manag. 70, 311-318 (1994). 8 Britton, A. J., Helliwell, R. C., Fisher, J. M. & Gibbs, S. Interactive effects of nitrogen deposition and fire on plant and soil chemistry in an alpine heathland. Environ. Poll, 156, 409-416 (2008). 9 Carreira, J. A. & Niell, F. X. Plant nutrient changes in a semi-arid Mediterranean shrubland after fire. J. Veg. Sci. 3, 457-466 (1992). 10 Christensen, N. Fire and soil-plant nutrient relations in a pine-wiregrass savanna on the coastal plain of North Carolina. Oecologia 31, 27-44 (1977). 11 Cui, Q. A., Lu, X. T., Wang, Q. B. & Han, X. G. Nitrogen fertilization and fire act independently on foliar stoichiometry in a temperate steppe. Plant Soil 334, 209-219 (2010). 12 DeByle, N. V., Urness, P. J. & Blank, D. L. Forage quality in burned and unburned aspen communities. USDA Forestry Service Research Paper INT-404 (1989). 13 Dorgeloh, W. G. Chemical quality of the burnt and non-burnt grass layer in the Nylsvlei Nature Reserve, South Africa. Afr. J. Ecol. 37, 168-179 (1999).
2 14 Duran, J., Rodriguez, A., Fernandez-Palacios, J. M. & Gallardo, A. Changes in leaf nutrient traits in a wildfire chronosequence. Plant Soil 331, 69-77 (2010). 15 Feeney, S. R., Kolb, T. E., Covington, W. W. & Wagner, M. R. Influence of thinning and burning restoration treatments on presettlement ponderosa pines at the Gus Pearson Natural Area. Can. J. For. Res. 28, 1295-1306 (1998). 16 Ferran, A., Delitti, W. & Vallejo, V. R. Effects of fire recurrence in Quercus coccifera L. shrublands of the Valencia Region (Spain): II. plant and soil nutrients. Plant Ecol. 177, 71-83 (2005). 17 FrancoVizcaino, E. & SosaRamirez, J. Soil properties and nutrient relations in burned and unburned Mediterranean-climate shrublands of Baja California, Mexico. Acta Oecol.-Int. J. Ecol. 18, 503-517 (1997). 18 Gilliam, F. S. Interactions of fire with nutrients in the herbaceous layer of a nutrient-poor coastal plain forest. Bull. Torrey Bot. Club 115, 265-271 (1988). 19 Gillon, D., Houssard, C., Valette, J. C. & Rigolot, E. Nitrogen and phosphorus cycling following prescribed burning in natural and managed Aleppo pine forests. Can. J. For. 29, 1237-1247 (1999). 20 Goergen, E. M. & Chambers, J. C. Influence of a native legume on soil N and plant response following prescribed fire in sagebrush steppe. Int. J. Wildland Fire 18, 665-675, (2009). 21 Hardtle, W. et al. Shifts in N and P budgets of heathland ecosystems: effects of management and atmospheric inputs. Ecosystems 12, 298-310 (2009). 22 Harris, G. R. & Covington, W. W. The effect of a prescribed fire on nutrient concentration and standing crop of understory vegetation in ponderosa pine. Can. J. For. Res. 13, 501-507 (1983). 23 Haskins, K. E. & Gehring, C. A. Interactions with juniper alter pinyon pine ectomycorrhizal fungal communities. Ecology 85, 2687-2692 (2004). 24 Henry, H. A. L., Chiariello, N. R., Vitousek, P. M., Mooney, H. A. & Field, C. B. Interactive effects of fire, elevated carbon dioxide, nitrogen deposition, and precipitation on a California annual grassland. Ecosystems 9, 1066-1075 (2006). 25 Huang, J. J. & Boerner, R. E. J. Effects of fire alone or combined with thinning on tissue nutrient concentrations and nutrient resorption in Desmodium nudiflorum. Oecologia 153, 233-243 (2007). 26 James, T. D. W. & Smith, D. W. Short-term effects of surface fire on the biomass and nutrient standing crop of Populus tremuloides in southern Ontario. Can. J. For. Res. 7, 666-679 (1977). 27 Jensen, M., Michelsen, A. & Gashaw, M. Responses in plant, soil inorganic and microbial nutrient pools to experimental fire, ash and biomass addition in a woodland savanna. Oecologia 128, 85-93 (2001). 28 Kituku, V. M., Powell, J., Smith, M. A. & Olson, R. A. Increasing bitterbrush nutrient quality with 2,4-D, mowing, and burning in south-central Wyoming. J. Range Manag. 45, 488-493 (1992). 29 Kranabetter, J. M. & Yole, D. Alternatives to broadcast burning in the northern interior of British Columbia: Short-term tree results. For. Chron. 76, 349-353 (2000). 30 Launonen, T. M., Ashton, D. H. & Keane, P. J. The effect of regeneration burns on the growth, nutrient acquisition and mycorrhizae of Eucalyptus regnans F. Muell. (mountain ash) seedlings. Plant Soil 210, 273-283 (1999). 31 Lloyd, P. S. Effects of fire on the chemical status of herbaceous communities of the Derbyshire Dales. J. Ecol. 59, 261-273 (1971).
3 32 Lu, X. T., Lu, F. M., Zhou, L. S., Han, X. & Han, X. G. Stoichiometric response of dominant grasses to fire and mowing in a semi-arid grassland. J. Arid Environ. 78, 154- 160 (2012). 33 McKevlin, M. R. & McKee Jr, W. H. Long-term prescribed burning increases nutrient uptake and growth of loblolly pine seedlings. For. Ecol. Manag. 17, 245-252 (1986). 34 Miesel, J. R. Differential responses of Pinus ponderosa and Abies concolor foliar characteristics and diameter growth to thinning and prescribed fire treatments. For. Ecol. Manag. 284, 163-173 (2012). 35 Miller, G. R. Quantity and quality of the annual production of shoots and flowers by Calluna vulgaris in North-East Scotland. J. Ecol. 67, 109-129 (1979). 36 Morley, S., Grant, C., Hobbs, R. & Cramer, V. Long-term impact of prescribed burning on the nutrient status and fuel loads of rehabilitated bauxite mines in Western Australia. For. Ecol. Manag. 190, 227-239 (2004). 37 Nardoto, G. B., Bustamante, M. M. D., Pinto, A. S. & Klink, C. A. Nutrient use efficiency at ecosystem and species level in savanna areas of Central Brazil and impacts of fire. J. Trop. Ecol. 22, 191-201 (2006). 38 Nzila, J. D., Bouillet, J. P., Laclau, J. P. & Ranger, J. The effects of slash management on nutrient cycling and tree growth in Eucalyptus plantations in the Congo. For. Ecol. Manag. 171, 209-221 (2002). 39 Oddvar, S., Wielgolaski, F. E. & Moe, B. Biomass and chemical composition of common forest plants in response to fire in western Norway. J. Veg. Sci. 9, 501-510 (1998). 40 Old, S. M. Microclimate, fire, and plant production in an Illinois prairie. Ecol. Monogr. 39, 355-384 (1969). 41 Osiname, O. A. & Meppe, F. Effects of different methods of plant residue management on soil properties and maize yield. Comm. Soil Sci. Plant Anal. 30, 53-63 (1999). 42 Payton, I. J., Lee, W. G., Dolby, R. & Mark, A. F. Nutrient concentrations in narrow- leaved snow tussock (Chionochloa rigida) after spring burning. New Zeal. J. Bot. 24, 529-537 (1986). 43 Pearson, H. A., Davis, J. R. & Schubert, G. H. Effects of wildfire on timber and forage production in Arizona. J. Range Manag. 25, 250-253 (1972). 44 Piatek, K. B. & Allen, H. L. Site preparation effects on foliar N and P use, retranslocation, and transfer to litter in 15-years old Pinus taeda. For. Ecol. Manag. 129, 143-152 (2000). 45 Rau, B. M., Chambers, J. C., Blank, R. R. & Johnson, D. W. Prescribed fire, soil, and plants: Burn effects and interactions in the central great basin. Rangeland Ecol. Manag. 61, 169-181 (2008). 46 Reich, P. B., Abrams, M. D., Ellsworth, D. S., Kruger, E. L. & Tabone, T. J. Fire affects ecophysiology and community dynamics of central Wisconsin oak forest regeneration. Ecology 71, 2179-2190 (1990). 47 Ross, D. J., Speir, T. W., Tate, K. R. & Feltham, C. W. Burning in a New Zealand snow- tussock grassland - Effects on soil microbial biomass and nitrogen and phosphorus availability. New Zeal. J. Ecol. 21, 63-71 (1997). 48 Rundel, P. W. & Parsons, D. J. Nutrient changes in two chaparral shrubs along a fire- induced age gradient. Am. J. Bot. 67, 51-58 (1980). 49 Schafer, J. L. & Mack, M. C. Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 334, 433-447 (2010). 50 Singh, R. S. Effect of winter fire on primary productivity and nutrient concentration of a dry tropical savanna. Vegetatio 106, 63-71 (1993). 51 Smith, E. F. & Young, V. A. The effect of burning on the chemical composition of little bluestem. J. Range Manag. 12, 139-140 (1959).
4 52 Uhl, C. & Jordan, C. F. Succession and nutrient dynamics following forest cutting and burning in Amazonia. Ecology 65, 1476-1490 (1984). 53 Van de Vijver, C. A. D. M., Poot, P. & Prins, H. H. T. Causes of increased nutrient concentrations in post-fire regrowth in an East African savanna. Plant Soil 214, 173-185 (1999). 54 von Oheimb, G. et al. Is Calluna vulgaris a suitable bio-monitor of management- mediated nutrient pools in heathland ecosystems? Ecol. Indic. 9, 1049-1055 (2009).
Appendix C: Comparison of Results from Meta-analyses Using Different Weighted Effect Sizes
Table S2. Comparison of Results from the Meta-analyses on Plant [N], [P] and N/P for All Observations
<4 Years Since Last Fire using WR and WU Weighted Effect Sizes
WR weighted WU weighted Nutrient Category N LnR (%) 95% CI LnR (%) 95% CI min max min max N N/P < 10 116 10.9 5.8 16.6 16.4 11.0 22.3 10 < N/P < 20 112 14.2 9.6 19.5 18.0 12.6 23.8 N/P > 20 28 -5.6 -20.2 10.5 1.2 -9.9 11.3
P N/P < 10 116 7.7 2.5 13.5 8.6 3.1 14.3 5 10 < N/P < 20 112 14.9 9.9 20.6 18.5 12.2 25.9 N/P > 20 28 63.3 29.0 103.4 46.9 25.4 73.7
N/P N/P < 10 116 3.0 -2.6 9.2 7.2 2.1 12.8 10 < N/P < 20 112 -0.6 -4.2 3.2 -0.5 -3.9 2.9 N/P > 20 28 -42.2 -57.1 -22.7 -31.2 -44.0 -17.1 Results are shown for the following categories: plant N/P in control plots < 10 (N/P < 10), plant N/P in control plots between 10 and 20 (10 < N/P < 20), and plant N/P in control plots > 20 (N/P > 20). Effects sizes (LnR) are shown in bold when 95% bootstrapped confidence intervals, 95% CI, did not overlap with 0.
Appendix D: Comparison of Results from Meta-analyses Using All Observations and One Random Observation per Study
Table S3. Summary of Results from the Meta-analyses on Plant [N], [P] and N/P Nutrient Category N LnR (%) 95% CI min max N All observations 256 10.5 6.7 14.5 1 random observation per study 47 11.1 5.5 17.9
P All observations 256 14.5 9.9 19.7 1 random observation per study 47 12.4 2.0 25.7
N/P All observations 256 -3.5 -8.3 1.1 1 random observation per study 47 -1.1 -10.1 8.5 Bold, when 95% bootstrapped confidence intervals, 95% CI, did not overlap with 0. Results are shown for all observations <4 years since last fire and for 1 random observation per study. Effects sizes (LnR) were weighted by WR.
6 Appendix E: Means and Standard Deviations of Plant [N], [P] and N/P in Control and Burned Plots
Table S4. Means ± Standard Deviations of Plant [N], [P] and N/P in Control and Burned Plots for Different Categories Category N (mg g-1) P (mg g-1) N/P N Control Burned Control Burned Control Burned All observations N/P < 10 116 12.2 ± 5.3 14.3 ± 6.6 2.05 ± 1.02 2.19 ± 1.02 6.4 ± 1.9 7.0 ± 2.6 10 < N/P < 20 112 15.7 ± 8.0 18.3 ± 8.4 1.18 ± 0.65 1.40 ± 0.77 13.8 ± 2.5 13.9 ± 3.7 N/P > 20 28 16.1 ± 7.9 16.0 ± 7.0 0.59 ± 0.35 0.90 ± 0.49 29.0 ± 8.9 21.0 ± 8.8 Herbaceous N/P < 10 87 12.0 ± 4.5 13.9 ± 6.0 2.02 ± 0.83 2.26 ± 1.04 6.2 ± 1.8 6.5 ± 2.3 10 < N/P < 20 45 17.0 ± 10.0 19.1 ± 9.9 1.33 ± 0.81 1.45 ± 0.79 13.2 ± 2.8 13.9 ± 4.4 N/P > 20 10 16.0 ± 10.3 17.1 ± 8.5 0.68 ± 0.50 0.81 ± 0.38 25.8 ± 7.6 23.5 ± 10.5 Woody N/P < 10 29 13.0 ± 7.3 15.7 ± 8.1 2.11 ± 1.48 1.97 ± 0.94 6.8 ± 2.0 8.3 ± 2.9 10 < N/P < 20 67 14.9 ± 6.2 17.7 ± 7.2 1.08 ± 0.51 1.37 ± 0.76 14.1 ± 2.3 13.9 ± 3.2 N/P > 20 18 16.2 ± 6.5 15.4 ± 6.2 0.54 ± 0.23 0.95 ± 0.54 30.8 ± 9.3 19.6 ± 7.6
Prescribed burn 200 14.8 ± 7.1 16.7 ± 7.7 1.56 ± 0.98 1.72 ± 0.96 12.0 ± 7.6 11.5 ± 5.7 Wildfire 53 11.9 ± 6.6 14.5 ± 7.5 1.29 ± 0.93 1.64 ± 1.11 12.7 ± 8.6 11.8 ± 8.0 Slash and burn 3 15.2 ± 2.8 17.3 ± 3.4 1.67 ± 0.32 1.97 ± 0.41 9.6 ± 3.9 9.3 ± 4.2
Species 196 15.1 ± 7.5 17.3 ± 8.0 1.47 ± 0.98 1.66 ± 0.93 12.8 ± 7.7 12.2 ± 5.7 Community 60 11.3 ± 4.5 12.9 ± 5.5 1.63 ± 0.94 1.85 ± 1.15 9.7 ± 7.6 9.5 ± 7.3 7 N-fixing 18 23.4 ± 10.6 25.3 ± 9.7 1.75 ± 0.77 1.80 ± 0.75 14.6 ± 5.5 15.6 ± 6.5 Non N-fixing 178 14.2 ± 6.6 16.4 ± 7.3 1.44 ± 1.00 1.64 ± 0.95 12.6 ± 7.9 11.8 ± 5.5 Results are shown for observations <4 years since last fire.
Appendix F: Meta-analysis results of the response of plant [N], [P] and N/P to fire for all observations
8 30 Herbaceous Woody (a) All (b) (c) )
d (132) e 20 (87) n (91) r (144) (45) (53) ] u
N (10) b [
n (32) t u n 10 (22) a o l t
p
d n e r o
a 0 e p r i f m
f o o c
t e
c -10 g e f n f a E h c
-20 % (
-30
) 140 (d) (e) (f) d e n r
] 120 u P b [
n t u n 100 a o l t
p
d n
e 80 r o
a e p r i
f 60 m
f o o c
t e
c 40 g e f n f a E
h 20 c
%
( 0
-20 (g) (h) (i) ) d
e 20 n r P u / b N n
t u
n 0 o a t l
p d
e n r o
a -20 e p r i f m
f o c o
t
e -40 c g e f n f a E h c -60 % (
-80 N/P<10 10 < N/P< 20 N/P>20 N/P<10 10 < N/P< 20 N/P>20 N/P<10 10 < N/P< 20 N/P>20
Plant N/P in unburned plots
Fig. S1. Meta-analysis results of the response of plant [N], [P] and N/P to fire for all 308 observations. Fire effects on plant [N] (a-c), plant [P] (d-f) and plant N/P (g-i) are shown for observations where plant N/P in unburned or pre-burned control plots were smaller than 10, between 10 and 20, and larger than 20. Results are shown for all observations (a, d, g), herbaceous plants (b, e, h) and woody plants (c, f, i). Effect sizes were weighted by replication. Error bars indicate bootstrapped 95% confidence intervals. The number of observations for each category is shown in brackets. Appendix G: Meta-analysis results of the response of plant [N], [P] and N/P to fire grouped by ecosystem type
9 a b c Temperate grassland (24)
Semiarid grassland (27)
Tropical grassland (8)
Heathland (28)
Shrubland (66)
Savanna (44)
Boreal forest (7)
Temperate forest (72)
Mediterranean forest (30)
Tropical forest (2)
-40 -20 0 20 40 60 80 100 -40 -20 0 20 40 60 80 -40 -20 0 20 40 60 Fire effect (% change compared to unburned)
Fig. S2. Meta-analysis results of the response of plant [N] (a), [P] (b) and N/P (c) to fire grouped by ecosystem type. Effect sizes were weighted by replication. Plant [N], [P] and N/P effect sizes were not significantly different among ecosystem types (Prandom = 0.36, 0.08 and 0.51 respectively). Error bars indicate bootstrapped 95% confidence intervals. The number of observations for each category is shown in brackets.
Appendix H: Effect sizes of plant [N], [P] and N/P as a function of effect sizes of available soil N, P, and N/P
10 1.5 a
1.0 ] N [
t n a
l 0.5 p
R n L
0.0
-0.5 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 LnR extractable soil N 1.5
b Shallow sampling depth Deep sampling depth 1.0 Unknown sampling depth ] P [
t n a l 0.5 p
R n L
0.0
-0.5 -2 -1 0 1 2 3 4 LnR extractable soil P 0.4 c 0.2 P /
N 0.0
t n a l p
R -0.2 n L
-0.4
-0.6 -3 -2 -1 0 1 2 LnR extractable soil N/P
Fig. S3. Effect sizes (natural log of the response ratio, LnR) of plant [N], [P] and N/P as a function of effect sizes of available soil N (a), P (b), and N/P (c) respectively, measured to less than 10 cm soil depth (shallow sampling depth), to 10 cm soil depth or deeper (up to 20 cm, deep sampling depth), and unknown sampling depth. No significant relationships were found.
11