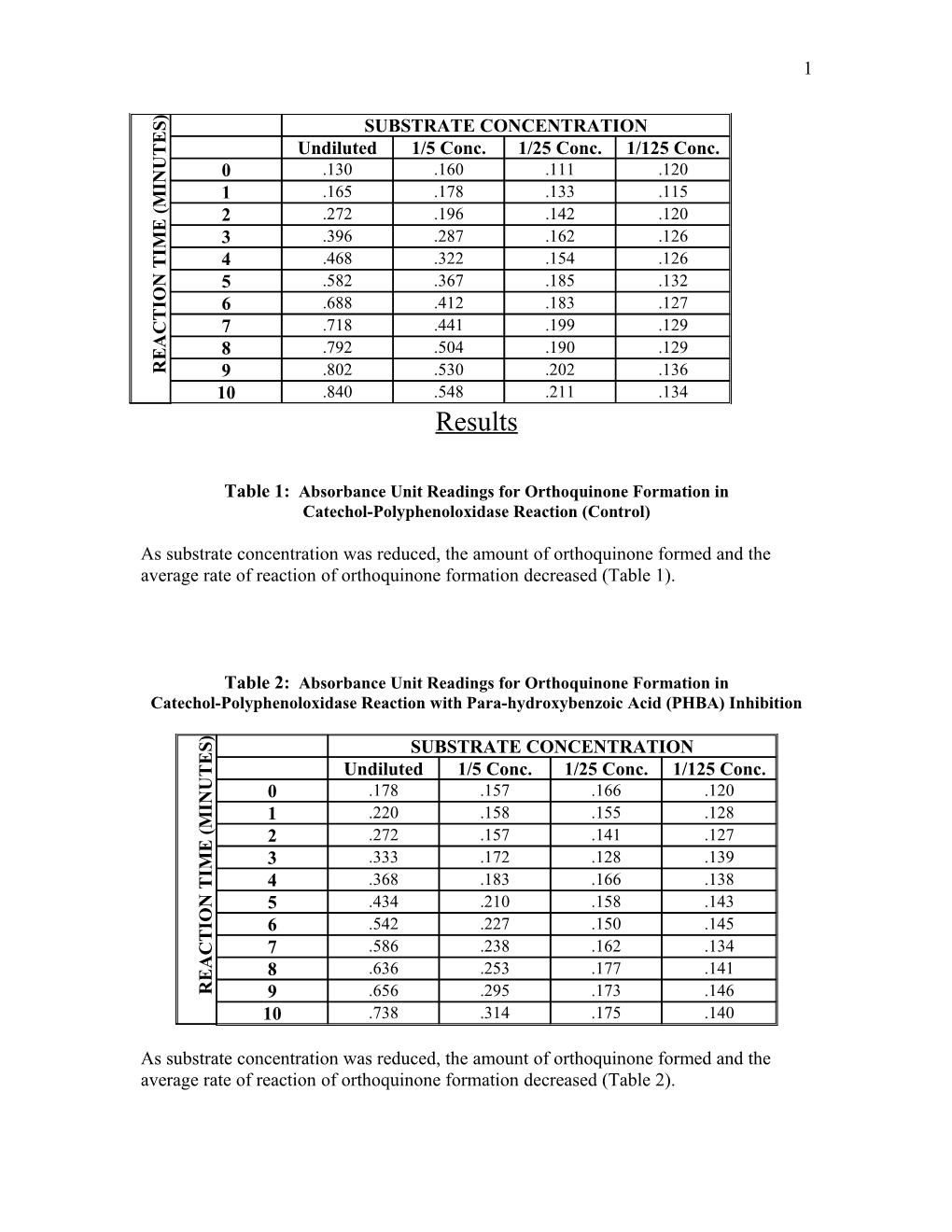
average rate of reaction of orthoquinone formation decreased (Table 1). (Table decreased oforthoquinone formation ofreaction rate average the formed oforthoquinone and amount reduced, was the substrate concentration As average rate of reaction of orthoquinone formation decreased (Table 2). (Table decreased oforthoquinone formation of reaction rate average the formed of orthoquinone and amount reduced, was the substrate concentration As Catechol-Polyphenoloxidase Reaction (PHBA)Catechol-Polyphenoloxidase with Inhibition Acid Para-hydroxybenzoic REACTION TIME (MINUTES)
REACTION TIME (MINUTES) 10 9 8 7 6 5 4 3 2 1 0 Table 2: Table 1: 10 3 2 1 0 9 8 7 6 5 4 Absorbance FormationUnit for Absorbance Orthoquinone Readings in FormationUnit for Absorbance Orthoquinone Readings in Undiluted Catechol-Polyphenoloxidase ReactionCatechol-Polyphenoloxidase(Control) .840 .802 .792 .718 .688 .582 .468 .396 .272 .165 .130 Undiluted SUBSTRATE CONCENTRATION .333 .272 .220 .178 .738 .656 .636 .586 .542 .434 .368 SUBSTRATE CONCENTRATION 1/5 Conc. .548 .530 .504 .441 .412 .367 .322 .287 .196 .178 .160 Results 1/5 Conc.1/5 .314 .295 .253 .238 .227 .210 .183 .172 .157 .158 .157 1/25 Conc.1/25 .211 .202 .190 .199 .183 .185 .154 .162 .142 .133 .111 1/25 Conc. .175 .173 .177 .162 .150 .158 .166 .128 .141 .155 .166 1/125 Conc. .134 .136 .129 .129 .127 .132 .126 .126 .120 .115 .120 1/125 Conc. .140 .146 .141 .134 .145 .143 .138 .139 .127 .128 .120 1 2
Table 3: Absorbance Unit Readings for Orthoquinone Formation in Catechol-Polyphenoloxidase Reaction with Potassium Arsenite Inhibition )
S SUBSTRATE CONCENTRATION E
T Undiluted 1/5 Conc. 1/25 Conc. 1/125 Conc.
U 0 .130 .123 .118 .096 N I 1 .223 .149 .122 .714 M ( 2 .326 .171 .128 .109 E 3 .438 .206 .140 .114 M I .540 .232 .153 .117
T 4
N 5 .620 .270 .157 .133 O
I 6 .676 .290 .169 .140 T .748 .340 .177 .153 C 7
A 8 .804 .368 .192 .153. E
R 9 .815 .400 .204 155 10 .835 .442 .213 .162
As substrate concentration was reduced, the amount of orthoquinone formed and the average rate of reaction of orthoquinone formation decreased (Table 3).
The control reaction and the reaction with PHBA acting as inhibitor had the same relative Vmax while the KM of the reaction with PHBA acting as inhibitor was greater than the control reaction (Fig.1). Due to possible error, the initial reaction rates of the reaction run with potassium arsenite as inhibitor are inconclusive. 3
Discussion
The experiments we carried out in part five of the enzyme action laboratory demonstrate the effectiveness of Para-hydroxybenzoic acid (PHBA) as a competitive inhibitor of the enzyme poyphenoloxidase. The experiments also prove that potassium arsenite is incapable of reducing orthoquinone formation. Both reactions with these two possible inhibitors are being compared to a control reaction involving neither PHBA, potassium arsenite, nor any other type of inhibitor.
Several conclusions can be drawn from the results of the control reaction and reaction run with PHBA as an inhibitor. The control reaction shows an upward trend in orthoquinone formation with time (Table 1) as does the reaction involving PHBA
(Table 2). But while both reactions have a similar Vmax (Fig.1), the KM of the reaction involving PHBA is much greater than the KM of the control reaction (Fig.1) and indicates that PHBA is acting as a competitive enzyme. The PHBA inhibits due to its structure. Because PHBA has a molecular design similar to catechol, it is able to enter the active site of polyphenoloxidase and block substrate-enzyme inhibition. The results of such blockage could have profound effects on the survival of an organism in nature.
If a plant such as the potato, which uses the catechol-polyphenoloxidase reaction as a defense mechanism, were to be exposed to an inhibitor such as PHBA, it would most likely die or be damaged due to predatory attack. This could possibly occur because the inhibitor, by blocking the active sites of polyphenoloxidase, will slow the formation of orthoquinones. The slow production of orthoquinone, a toxic repellent, may provide predators such as microbes, insects, or fungi with more time to damage the plant and consume vital starches and proteins. Thus, as in the case of the potato and PHBA, it is highly dangerous to allow certain plants to be exposed to possible competitive enzymatic 4 inhibitors. The introduction of competitive inhibitors into an ecosystem could increase the amount of unwanted predators and likewise decrease vital plant life.
Potassium arsenite, as an inhibitor, has little or no negative affect on the rate of catechol-polyphenoloxidase reaction (Fig.1) or amount of orthoquinone formation
(Table 2). Because potassium arsenite affects the disulfide bonds of enzymes, it may be concluded that the breaking of disulfide bonds of polyphenoloxidase has no affect on the conformation of its active site. Were the shape of the active site to be altered, then product formation and rate of reaction would have both been decreased. There may not be enough disulfide bonds holding the tertiary structure of polyphenoloxidase together for potassium arsenite to sufficiently denature the enzyme. Also, the number of other structures holding the tertiary structure of polyphenoloxidase together (hydrogen bonds, ionic/covalent bonds, Van der Waals attractions) may be strong enough to hold the enzyme together regardless of the affect of potassium arsenite on disulfide bonds.
Therefore, if an inhibitor such as potassium arsenite were to interact with a plant species such as the potato in the wild, there would presumably be no negative affect on the potato. However, the addition of potassium arsenite could make the potato more susceptible to denaturization from other sources in nature.