Supplementary material
Tables
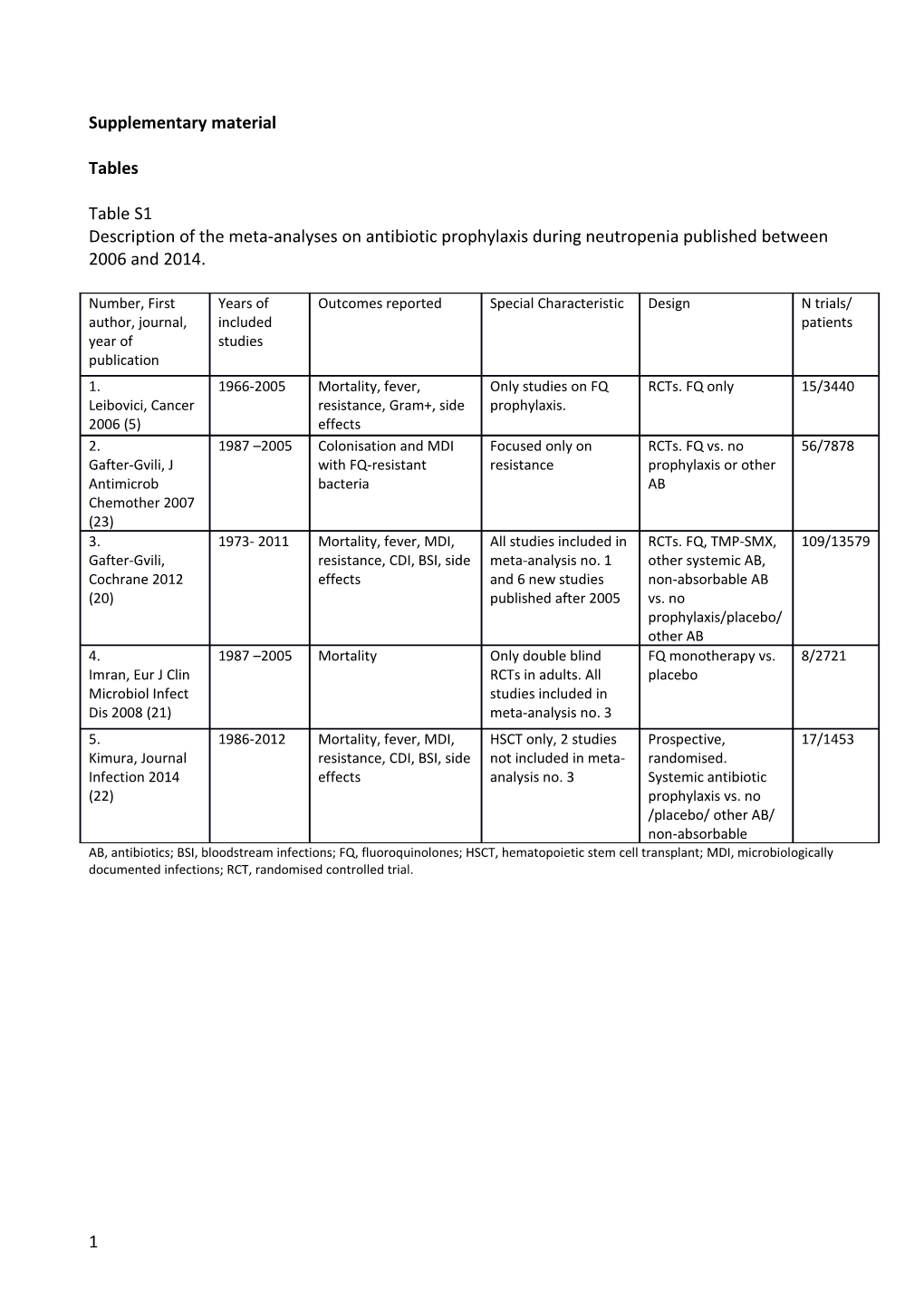
Table S1 Description of the meta-analyses on antibiotic prophylaxis during neutropenia published between 2006 and 2014.
Number, First Years of Outcomes reported Special Characteristic Design N trials/ author, journal, included patients year of studies publication 1. 1966-2005 Mortality, fever, Only studies on FQ RCTs. FQ only 15/3440 Leibovici, Cancer resistance, Gram+, side prophylaxis. 2006 (5) effects 2. 1987 –2005 Colonisation and MDI Focused only on RCTs. FQ vs. no 56/7878 Gafter-Gvili, J with FQ-resistant resistance prophylaxis or other Antimicrob bacteria AB Chemother 2007 (23) 3. 1973- 2011 Mortality, fever, MDI, All studies included in RCTs. FQ, TMP-SMX, 109/13579 Gafter-Gvili, resistance, CDI, BSI, side meta-analysis no. 1 other systemic AB, Cochrane 2012 effects and 6 new studies non-absorbable AB (20) published after 2005 vs. no prophylaxis/placebo/ other AB 4. 1987 –2005 Mortality Only double blind FQ monotherapy vs. 8/2721 Imran, Eur J Clin RCTs in adults. All placebo Microbiol Infect studies included in Dis 2008 (21) meta-analysis no. 3 5. 1986-2012 Mortality, fever, MDI, HSCT only, 2 studies Prospective, 17/1453 Kimura, Journal resistance, CDI, BSI, side not included in meta- randomised. Infection 2014 effects analysis no. 3 Systemic antibiotic (22) prophylaxis vs. no /placebo/ other AB/ non-absorbable AB, antibiotics; BSI, bloodstream infections; FQ, fluoroquinolones; HSCT, hematopoietic stem cell transplant; MDI, microbiologically documented infections; RCT, randomised controlled trial.
1 Table S2 Available guidelines on prophylaxis in patients with haematological malignancies.
Author, year of Country/ group FQ prophylaxis Start and end of prophylaxis Prophylaxis not recommended Agent against Gram- publication recommended positives Bucaneve 2007 ECIL High risk: neutropenia ≥ 7 Start with chemotherapy, only FQ ND ND (1) days (AI for levofloxacin prophylaxis start 24–48 h after the end of and ciprofloxacin) high dose cyclophosphamide therapy (AIII), and continue until resolution of neutropenia or initiation of empirical antibacterial therapy for febrile neutropenia (AII) Freifeld 2011 IDSA High risk: neutropenia ≥ 7 With the first day of cytotoxic therapy or Low-risk patients who are Not recommended (A-I) (24) days, BI the day following administration of the last anticipated to remain dose of chemotherapy, stop at the end of neutropenic for <7 days (A-III) neutropenia Slavin 2011 Australian Consider in outpatient ND Not be routinely used in high- ND (25) HSCT and palliative risk haematology patients patients with bone (grade C); Low risk of marrow failure (grade C) developing neutropenic fever (<7days, mostly solid tumours) (level I-II, grade C) Phillips 2012 UK/NICE Adult patients (aged ≥18 During the expected period of neutropenia ND ND (26) years) with acute leukaemia, HSCT, or solid tumours (duration of n/p not mentioned) Baden NCCN High risk: HSCT, acute ND Neutropenia expected to last Consider penicillin and 2012/2013 (29) leukaemia, lymphoma, less than 7 days who are not TMP-SMX for allogeneic multiple myeloma, receiving immunosuppressive HSCT recipients with alemtuzumab, GvHD, regimens (e.g., systemic GvHD neutropenia ≥ 7 days corticosteroids) Flowers 2013 ASCO High risk: neutropenia for ND If neutropenia is less severe or ND (27) > 7 days, unless other of shorter duration and the factors which increase usual course with current risks for complications or chemotherapy regimens for mortality solid tumours Neumann 2013 Germany (AGIHO, DGHO) High risk: neutropenia ≥ 7 Start prophylaxis with onset of Low risk except for those Not recommended (E-II) (28) days; some low risk (1st neutropenia in low-risk patients, with start mentioned in the chemo, aggressive chemo of cytostatic drugs in high-risk patients "recommended" group with high infections rate, (both BIII) elderly)
2 Klastersky European/ ESMO Never: the use of NA All patients with febrile Discouraged. 2016 (30) antimicrobials, including neutropenia. fluoroquinolones, should be discouraged.
3 Table S3 PRISMA checklist
Section/topic # Checklist item Reported on page #
TITLE
Title 1 Identify the report as a systematic review, meta-analysis, or both. Not reported. Systematic review and meta- regression were tools for the critical appraisal reported in the title ABSTRACT
Structured summary 2 Provide a structured summary including, as applicable: background; objectives; data sources; study 3 eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. INTRODUCTION
Rationale 3 Describe the rationale for the review in the context of what is already known. 4 Objectives 4 Provide an explicit statement of questions being addressed with reference to participants, interventions, 4 comparisons, outcomes, and study design (PICOS). METHODS
Protocol and registration 5 Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, 5 provide registration information including registration number. Eligibility criteria 6 Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years 5 considered, language, publication status) used as criteria for eligibility, giving rationale. Information sources 7 Describe all information sources (e.g., databases with dates of coverage, contact with study authors to 5 identify additional studies) in the search and date last searched. Search 8 Present full electronic search strategy for at least one database, including any limits used, such that it Only keywords for research were provided since could be repeated. the search was performed independently by three authors. Study selection 9 State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if 5, 7 applicable, included in the meta-analysis). Data collection process 10 Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and 5 any processes for obtaining and confirming data from investigators. Data items 11 List and define all variables for which data were sought (e.g., PICOS, funding sources) and any 6 assumptions and simplifications made.
4 Risk of bias in individual studies 12 Describe methods used for assessing risk of bias of individual studies (including specification of whether 7 this was done at the study or outcome level), and how this information is to be used in any data synthesis. Summary measures 13 State the principal summary measures (e.g., risk ratio, difference in means). 6 Synthesis of results 14 Describe the methods of handling data and combining results of studies, if done, including measures of 6 consistency (e.g., I2) for each meta-analysis. Risk of bias across studies 15 Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, 6 selective reporting within studies). Additional analyses 16 Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, 6,7 indicating which were pre-specified. RESULTS
Study selection 17 Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for Figures S1, S2 exclusions at each stage, ideally with a flow diagram. Study characteristics 18 For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up Tables 1-4 period) and provide the citations. Risk of bias within studies 19 Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). Table 3 Results of individual studies 20 For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for Table 1 and 4, figures 1,2,3 and S3, S4, S5 each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. Synthesis of results 21 Present results of each meta-analysis done, including confidence intervals and measures of consistency. Risk of bias across studies 22 Present results of any assessment of risk of bias across studies (see Item 15). 11 Additional analysis 23 Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Table S3, figure 4 and figure S6 Item 16]). DISCUSSION
Summary of evidence 24 Summarise the main findings including the strength of evidence for each main outcome; consider their 13, 14,15 relevance to key groups (e.g., healthcare providers, users, and policy makers). Limitations 25 Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete 15,16 retrieval of identified research, reporting bias). Conclusions 26 Provide a general interpretation of the results in the context of other evidence, and implications for 15,16 future research. FUNDING
Funding 27 Describe sources of funding for the systematic review and other support (e.g., supply of data); role of 18 funders for the systematic review. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097 5 Table S4 Results of meta-regressions on the impact of the prevalence of background FQ resistance in community and in hospital settings on the efficacy of FQ prophylaxis defined as the rate of mortality, bloodstream infections and episodes of fever during neutropenia.
Outcome N. of Percent 95% Confidence P value studies change % Interval FQ resistance in community setting RCT Mortality 9 -3.5 -10.5 / 4.2 0.310 BSI 10 2.5 -2.8 / 8.0 0.314 FN 11 -0.5 -6.2 / 5.5 0.852
Observational Mortality 7 -9.6 -33.0 / 22.0 0.427 studies BSI 9 -3.3 -11.9 / 6.2 0.424 FN 4 -13.2 -32.2 / 11.1 0.132 FQ resistance in hospital setting RCT Mortality 9 -3.6 -9.9 / 3.1 0.235 BSI 10 0.9 -3.0 / 5.0 0.612 FN 11 0.2 -4.8 / 5.4 0.947
Observational Mortality 7 -0.4 -7.4 / 7.2 0.904 studies BSI 9 2.0 -2.6 / 6.9 0.343 FN 4 -10.2 -27.5 / 11.1 0.161
* FQ resistance in a general population (1% unit change). BSI, bloodstream infection; FN, episodes of fever during neutropenia; RCT, randomised controlled trials. Percent change > 0 means that a 1% increase in the prevalence of background FQ resistance reduces the efficacy of FQ prophylaxis by percent change (%.)
6 Figures Figure S1 Review of randomised controlled trials (RCT), observational studies published between 2006 and 2014.
7 Figure S2 PRISMA flow diagram of studies included in meta-regressions.
Figure S3 Forrest plot on the effect of FQ prophylaxis on the overall mortality in randomised controlled trials (RCT) included in the meta-regressions.
8 Figure S4 Forrest plot on the effect of FQ prophylaxis on the rate of bloodstream infections in randomised controlled trials (RCT) included in the meta-regressions.
9 Figure S5 Forrest plot on the effect of FQ prophylaxis on the rate of episodes of fever during neutropenia in randomised controlled trials (RCT) included in the meta-regressions.
10 Figure S6
Line graph of the fitted values estimated from meta-regression of observational studies published after 2005 on the impact of the background FQ resistance in the community (left) and in the hospital (right) setting on the efficacy of FQ prophylaxis on the three endpoints (overall mortality, rate of bloodstream infections and rate of fever during neutropenia) plotted against the % of FQ resistance, together with the estimates from each study represented by circles. The circle size depends on the weight given to each study in the random-effects model.
FQ, fluoroquinolones; BSI, bloodstream infection; FN, episodes of fever during neutropenia. PC, percent change in OR when FQ increases of 1 unit, in brackets the 95% confidence interval. PC > 0 means that a 1% increase in the prevalence of background FQ resistance reduces the efficacy of FQ prophylaxis by PC %; Dashed line (OR=1): no effect of prophylaxis.
11