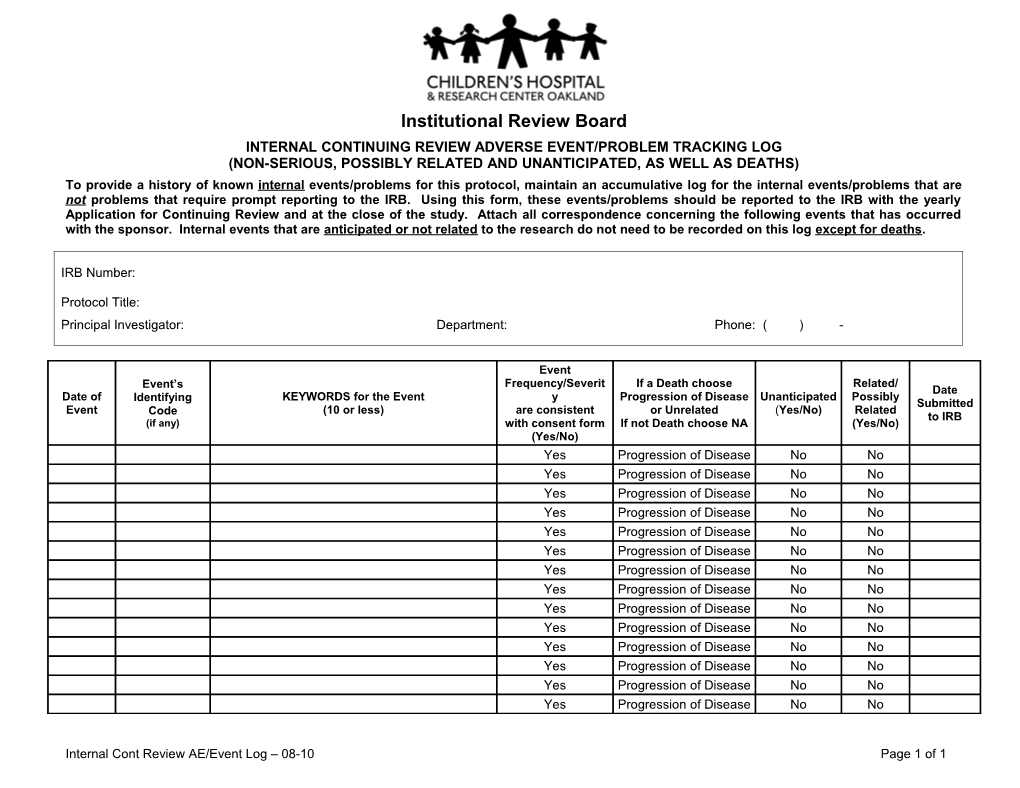
Institutional Review Board INTERNAL CONTINUING REVIEW ADVERSE EVENT/PROBLEM TRACKING LOG (NON-SERIOUS, POSSIBLY RELATED AND UNANTICIPATED, AS WELL AS DEATHS) To provide a history of known internal events/problems for this protocol, maintain an accumulative log for the internal events/problems that are not problems that require prompt reporting to the IRB. Using this form, these events/problems should be reported to the IRB with the yearly Application for Continuing Review and at the close of the study. Attach all correspondence concerning the following events that has occurred with the sponsor. Internal events that are anticipated or not related to the research do not need to be recorded on this log except for deaths.
IRB Number:
Protocol Title: Principal Investigator: Department: Phone: ( ) -
Event Event’s Frequency/Severit If a Death choose Related/ Date Date of Identifying KEYWORDS for the Event y Progression of Disease Unanticipated Possibly Submitted Event Code (10 or less) are consistent or Unrelated (Yes/No) Related to IRB (if any) with consent form If not Death choose NA (Yes/No) (Yes/No) Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No Yes Progression of Disease No No
Internal Cont Review AE/Event Log – 08-10 Page 1 of 1