Adapted Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In This Issue Birds SA Aims To: • Promote the Conservation of a SPECIAL BUMPER CHRISTMAS Australian Birds and Their Habitats
Linking people with birds in South Australia The Birder No 240 November 2016 In this Issue Birds SA aims to: • Promote the conservation of A SPECIAL BUMPER CHRISTMAS Australian birds and their habitats. ISSUE — LOTS OF PHOTOS • Encourage interest in, and develop knowledge of, the birds of South Australia. PLEASE VOLUNTEER — THE BIRDS • Record the results of research into NEED YOUR HELP! all aspects of bird life. • Maintain a public fund called the A NATIONAL PARK IN THE “Birds SA Conservation Fund” for INTERNATIONAL BIRD SANCTUARY the specific purpose of supporting the Association’s environmental objectives. CO N T E N T S N.B. ‘THE BIRDER’ will not be President’s Message 3 published in February 2017. The Birds SA Notes & News 4 next issue of this newsletter will be The Laratinga Birdfair 8 distributed at the March General Kangaroos at Sandy Creek CP. 9 Meeting, on 31 March 2017. Return of the Adelaide Rosella 10 Giving them wings 11 Cover photo Past General Meetings 13 Emu, photographed by Barbara Bansemer in Future General Meetings 15 Brachina Gorge, Flinders Ranges, on 26th Past Excursions 16 October 2016. Future Excursions 23 Bird Records 25 New Members From the Library 28 We welcome 25 new members who have About our Association 30 recently joined the Association. Their names are listed on p29. Photos from Members 31 CENTRE INSERT: SAOA HISTORICAL SERIES No: 58, JOHN SUTTON’S OUTER HARBOR NOTES, PART 8 DIARY The following is a list of Birds SA activities for the next few months. Further details of all these activities can be found later in ‘The Birder’. -
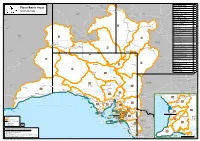
ACT Flood Watch Areas
! ! ! ! Barkly Warrego Flood Watch Area No. ! Homestead Ayr ! ! Tennant Roadhouse Angas and Brem!er Rivers 28 Flood Watch Areas ! Home Balgo Creek Camooweal Charters ! Broughton RivHeirll 19 ! Hill !Towers South Australia ! Cooper Creek Bowen 7 ! Mount Julia Danggali Rivers and CCreolelinkssville Prose1rp7ine Creek ! NT Isa Cloncurry ! ! ! Diamantina River 3 !Hughenden Eastern Eyre Peninsula 18 Stamford MACKAY " Telfer ! Eastern Great Victoria Desert 9 ! Sarina ! Finke River and Stephenson Creek 5 Fleurieu Peninsula 29 !Moranbah Flinders Ranges Rivers Cotton Yuendumu 1 ! Winton 15 Creek ! and Creeks ! Dysart Gawler River ! 24 Clermont Boulia ! ! Georgina River and Eyre Creek 1 Papunya ! Kangaroo Island 30 !Longreach Alice Lake Eyre !Emerald 10 Springs !Alpha WA ! 2 Lake Frome 11 Hermannsburg ! Santa 3 Lake Gairdner Springsure 12 !Teresa ! Light and Wakefield Rivers 21 Docker River QLD Limestone and (Kaltukatjara) 31 ! M!iTllaicmebnot Coast Rivers and Creeks Erldunda Lower Eyre Peninsula 22 ! ! Yulara Tourist Village 4 Windorah ! North West Lake Torrens 14 ! Carnegie NullarbAougr aDthiesltlarict Rivers 13 ! Birdsville ! ! Onkaparinga River 27 Warburton! ! River Murray Murraylands 26 Amata 5 Charleville ! Mitchell River Murray Riverlands! Rom20a !Quilpie ! Simpson Desert 4 Tjukayirla 6 Roadhouse Torrens and metropolitan rivers Surat ! 2!5 and creeks ! 7 Warburton District Rivers 6 Innamincka Oodnadatta 8 ! Warburton River 8 !Thargomindah St George West Coast Rivers and Creeks ! 16 Ilkurlka ! 9 Western Desert 2 Dirranbandi !Laverton ! Coober -

DUCK HUNTING in VICTORIA 2020 Background
DUCK HUNTING IN VICTORIA 2020 Background The Wildlife (Game) Regulations 2012 provide for an annual duck season running from 3rd Saturday in March until the 2nd Monday in June in each year (80 days in 2020) and a 10 bird bag limit. Section 86 of the Wildlife Act 1975 enables the responsible Ministers to vary these arrangements. The Game Management Authority (GMA) is an independent statutory authority responsible for the regulation of game hunting in Victoria. Part of their statutory function is to make recommendations to the relevant Ministers (Agriculture and Environment) in relation to open and closed seasons, bag limits and declaring public and private land open or closed for hunting. A number of factors are reviewed each year to ensure duck hunting remains sustainable, including current and predicted environmental conditions such as habitat extent and duck population distribution, abundance and breeding. This review however, overlooks several reports and assessments which are intended for use in managing game and hunting which would offer a more complete picture of habitat, population, abundance and breeding, we will attempt to summarise some of these in this submission, these include: • 2019-20 Annual Waterfowl Quota Report to the Game Licensing Unit, New South Wales Department of Primary Industries • Assessment of Waterfowl Abundance and Wetland Condition in South- Eastern Australia, South Australian Department for Environment and Water • Victorian Summer waterbird Count, 2019, Arthur Rylah Institute for Environmental Research As a key stakeholder representing 17,8011 members, Field & Game Australia Inc. (FGA) has been invited by GMA to participate in the Stakeholder Meeting and provide information to assist GMA brief the relevant Ministers, FGA thanks GMA for this opportunity. -

PETER EGERTON WARBURTON 1813-1889 British Military Officer; Commissioner of Police for South Australia; Australian Explorer
PETER EGERTON WARBURTON 1813-1889 British military officer; Commissioner of Police for South Australia; Australian explorer Kath Gee This article began as simple fact-finding for a walk around Norley: A Tale of Two Manors, which was part of the Frodsham Festival of Walks 2017. Peter Egerton Warburton was the 7th of 10 children of Reverend Rowland Egerton and his wife, Emma. Emma was the only daughter of James and Emma Croxton. Their family home was Norley Bank, one of two large estates in Norley, Cheshire. 19th century maps and documents convey the extent of the estate and historic images show the size and character of the house. FDN0489 Norley Bank house, south side (undated). Sadly, it was demolished in 1957 Emma Croxton was a sister of Sir Peter Warburton, 5th Baronet of Arley. When Sir Peter died without issue on the 14th May 1813, Rev’d Rowland and Emma were living at Norley Bank House. Their first child, Rowland Eyles Warburton, aged 7, inherited the estates of Arley and Warburton, and Rev’d Rowland and Emma were granted royal permission to append the name ‘Warburton’ to their own. It is, perhaps, unsurprising that their next child & 4th son, born on 15th August 1813, was christened Peter. It is aspects of Peter Egerton Warburton’s life that I have researched and presented here. Peter was educated at home and by tutors in France until the age of 12 when he entered the Royal Navy. Peter served as a Midshipman on HMS Windsor Castle (1) for the next 3 years. Then, in 1829 he entered the Royal Indian Military College at Addiscombe, Surrey, and on 9th June 1831 he became an ensign (2) in the 13th Native Infantry Battalion, in Bombay. -

Distribution and Habitats of the Grey Grasswren in South Australia
Government of South Australia South Australian Arid Lands Natural Resources Management Board October 2009 South Australian Arid Lands Natural Resources Management Board Distribution and habitats of the Grey Grasswren Amytornis barbatus in South Australia Andrew Black, Graham Carpenter, Lynn Pedler, Peter Langdon & Reece Pedler DISCLAIMER The South Australian Arid Lands Natural Resources Management Board, and its employees do not warrant or make any representation regarding the use, or results of use of the information contained herein as to its correctness, accuracy, reliability, currency or otherwise. The South Australian Arid Lands Natural Resources Management Board and its employees expressly disclaim all liability or responsibility to any person using the information or advice. © South Australian Arid Lands Natural Resources Management Board 2010 This work is copyright. Apart from any use permitted under the Copyright Act 1968 (Commonwealth), no part may be reproduced by any process without prior written permission obtained from the South Australian Arid Lands Natural Resources Management Board. Requests and enquiries concerning reproduction and rights should be directed to the General Manager, South Australian Arid Lands Natural Resources Management Board Railway Station Building, PO Box 2227, Port Augusta, SA, 5700 INTRODUCTION The Grey Grasswren (Amytornis barbatus) is a small elusive bird of floodplain habitats in inland river systems dominated by Lignum Muhlenbeckia cunninghamii (Higgins et al. 2001). The species is relatively new to science, being first seen in 1921 on the Bulloo floodplain on the Queensland/New South Wales border (Chenery 1922, Macgillivray 1923, McAllan 2000), seen again in that area in 1942 and 1967 by Norman Favaloro and described from specimens taken soon afterwards (Favaloro and McEvey 1968, Robinson 1973). -

Patterns of Evolution in Gobies (Teleostei: Gobiidae): a Multi-Scale Phylogenetic Investigation
PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE BS, Hofstra University, 2007 MS, Texas A&M University-Corpus Christi, 2010 Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in MARINE BIOLOGY Texas A&M University-Corpus Christi Corpus Christi, Texas December 2014 © Luke Michael Tornabene All Rights Reserved December 2014 PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE This dissertation meets the standards for scope and quality of Texas A&M University-Corpus Christi and is hereby approved. Frank L. Pezold, PhD Chris Bird, PhD Chair Committee Member Kevin W. Conway, PhD James D. Hogan, PhD Committee Member Committee Member Lea-Der Chen, PhD Graduate Faculty Representative December 2014 ABSTRACT The family of fishes commonly known as gobies (Teleostei: Gobiidae) is one of the most diverse lineages of vertebrates in the world. With more than 1700 species of gobies spread among more than 200 genera, gobies are the most species-rich family of marine fishes. Gobies can be found in nearly every aquatic habitat on earth, and are often the most diverse and numerically abundant fishes in tropical and subtropical habitats, especially coral reefs. Their remarkable taxonomic, morphological and ecological diversity make them an ideal model group for studying the processes driving taxonomic and phenotypic diversification in aquatic vertebrates. Unfortunately the phylogenetic relationships of many groups of gobies are poorly resolved, obscuring our understanding of the evolution of their ecological diversity. This dissertation is a multi-scale phylogenetic study that aims to clarify phylogenetic relationships across the Gobiidae and demonstrate the utility of this family for studies of macroevolution and speciation at multiple evolutionary timescales. -

Resistance and Resilience of Murray-Darling Basin Fishes to Drought Disturbance
Resistance and Resilience of Murray- Darling Basin Fishes to Drought Disturbance Dale McNeil1, Susan Gehrig1 and Clayton Sharpe2 SARDI Publication No. F2009/000406-1 SARDI Research Report Series No. 602 SARDI Aquatic Sciences PO Box 120 Henley Beach SA 5022 April 2013 Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 “Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations” McNeil et. al. 2013 Drought and Native Fish Resilience Resistance and Resilience of Murray- Darling Basin Fishes to Drought Disturbance Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 “Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations” Dale McNeil1, Susan Gehrig1 and Clayton Sharpe2 SARDI Publication No. F2009/000406-1 SARDI Research Report Series No. 602 April 2013 Page | ii McNeil et. al. 2013 Drought and Native Fish Resilience This Publication may be cited as: McNeil, D. G., Gehrig, S. L. and Sharpe, C. P. (2013). Resistance and Resilience of Murray-Darling Basin Fishes to Drought Disturbance. Final Report to the Murray-Darling Basin Authority - Native Fish Strategy Project MD/1086 ―Ecosystem Resilience and the Role of Refugia for Native Fish Communities & Populations‖. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000406-1. SARDI Research Report Series No. 602. 143pp. Front Cover Images – Lake Brewster in the Lower Lachlan River catchment, Murray-Darling Basin during extended period of zero inflows, 2007. Murray cod (Maccullochella peelii peelii), olive perchlet (Ambassis agassizii) and golden perch (Macquaria ambigua) from the, lower Lachlan River near Lake Brewster, 2007 (all images - Dale McNeil). -

Multi-Locus Fossil-Calibrated Phylogeny of Atheriniformes (Teleostei, Ovalentaria)
Molecular Phylogenetics and Evolution 86 (2015) 8–23 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Multi-locus fossil-calibrated phylogeny of Atheriniformes (Teleostei, Ovalentaria) Daniela Campanella a, Lily C. Hughes a, Peter J. Unmack b, Devin D. Bloom c, Kyle R. Piller d, ⇑ Guillermo Ortí a, a Department of Biological Sciences, The George Washington University, Washington, DC, USA b Institute for Applied Ecology, University of Canberra, Australia c Department of Biology, Willamette University, Salem, OR, USA d Department of Biological Sciences, Southeastern Louisiana University, Hammond, LA, USA article info abstract Article history: Phylogenetic relationships among families within the order Atheriniformes have been difficult to resolve Received 29 December 2014 on the basis of morphological evidence. Molecular studies so far have been fragmentary and based on a Revised 21 February 2015 small number taxa and loci. In this study, we provide a new phylogenetic hypothesis based on sequence Accepted 2 March 2015 data collected for eight molecular markers for a representative sample of 103 atheriniform species, cover- Available online 10 March 2015 ing 2/3 of the genera in this order. The phylogeny is calibrated with six carefully chosen fossil taxa to pro- vide an explicit timeframe for the diversification of this group. Our results support the subdivision of Keywords: Atheriniformes into two suborders (Atherinopsoidei and Atherinoidei), the nesting of Notocheirinae Silverside fishes within Atherinopsidae, and the monophyly of tribe Menidiini, among others. We propose taxonomic Marine to freshwater transitions Marine dispersal changes for Atherinopsoidei, but a few weakly supported nodes in our phylogeny suggests that further Molecular markers study is necessary to support a revised taxonomy of Atherinoidei. -

Systematic Taxonomy and Biogeography of Widespread
Systematics and Biogeography of Three Widespread Australian Freshwater Fish Species. by Bernadette Mary Bostock B.Sc. (Hons) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Deakin University February 2014 i ABSTRACT The variation within populations of three widespread and little studied Australian freshwater fish species was investigated using molecular genetic techniques. The three species that form the focus of this study are Leiopotherapon unicolor, Nematalosa erebi and Neosilurus hyrtlii, commonly recognised as the three most widespread Australian freshwater fish species, all are found in most of the major Australian drainage basins with habitats ranging from clear running water to near stagnant pools. This combination of a wide distribution and tolerance of a wide range of ecological conditions means that these species are ideally suited for use in investigating phylogenetic structure within and amongst Australian drainage basins. Furthermore, the combination of increasing aridity of the Australian continent and its diverse freshwater habitats is likely to promote population differentiation within freshwater species through the restriction of dispersal opportunities and localised adaptation. A combination of allozyme and mtDNA sequence data were employed to test the null hypothesis that Leiopotherapon unicolor represents a single widespread species. Conventional approaches to the delineation and identification of species and populations using allozyme data and a lineage-based approach using mitochondrial 16S rRNA sequences were employed. Apart from addressing the specific question of cryptic speciation versus high colonisation potential in widespread inland fishes, the unique status of L. unicolor as both Australia’s most widespread inland fish and most common desert fish also makes this a useful species to test the generality of current biogeographic hypotheses relating to the regionalisation of the Australian freshwater fish fauna. -

Riparian Vegetation of the River Murray COVER: Healthy Red Gum in the Kex)Ndrook State Forest Near Barham N.S.W
Riparian Vegetation of The River Murray COVER: Healthy red gum in the Kex)ndrook State Forest near Barham N.S.W. Background, black box silhouette. PHOTO: D. Eastburn ISBN 1 R75209 02 6 RIVER MURRAY RIPARIAN VEGET ION STUDY PREPARED FOR: MURRAY-DARLING BASIN COMMISSION BY: MARGULES AND PARTNERS PTY LTD PAND J SMITH ECOLOGICAL CONSULTANTS DEPARTMENT OF CONSERVATION FORESTS AND LANDS VICTORIA January 1990 SUMMARY AND CONCLUSIONS The River Murray Riparian Vegetation Survey was initiated by the Murray Darling Basin Commission t9 assessJhe present status ofthe vegetationalong the Murray, to identify causes ofdegradation, and to develop solutions for its rehabilitation and long term stability. The study area was the floodplain of the Murray River and its anabranches, including the Edward-Wakool system, from below Hume Dam to the upper end of Lake Alexandrina. The components of the study were: · Literature Review A comprehensive bibliography was compiled on the floodplain vegeta tion, its environment and the impact ofman's activities. The literature was reviewed and summarised. · Floristic Survey A field survey was carried out, visiting 112 sites throughout the study area and collecting vegetation data from 335 plots. Data collected were the species present, their relative abundance, the condition of the eucalypts, the amount ofeucalypt regeneration and indices ofgrazing pressure. Brief studies were made of the effects of river regulation and salinisation at specific sites. Thirty-seven plant communities were identified from a numerical analyis ofthe floristic survey data. The differences reflect environmental changes both along the river and across the floodplain. The most important factors were identified as soil salinity levels and flooding frequency. -

GAMBUSIA CONTROL in SPRING WETLANDS Final Report, October 2009
Government of South Australia South Australian Arid Lands Natural Resources Management Board October 2009 South Australian Arid Lands Natural Resources Management Board Gambusia control in spring wetlands Adam Kerezsy GAMBUSIA CONTROL IN SPRING WETLANDS Final report, October 2009 Adam Kerezsy Aquatic Ecologist, Bush Heritage Australia DISCLAIMER The South Australian Arid Lands Natural Resources Management Board, and its employees do not warrant or make any representation regarding the use, or results of use of the information contained herein as to its correctness, accuracy, reliability, currency or otherwise. The South Australian Arid Lands Natural Resources Management Board and its employees expressly disclaim all liability or responsibility to any person using the information or advice. © South Australian Arid Lands Natural Resources Management Board 2009 This work is copyright. Apart from any use permitted under the Copyright Act 1968 (Commonwealth), no part may be reproduced by any process without prior written permission obtained from the South Australian Arid Lands Natural Resources Management Board. Requests and enquiries concerning reproduction and rights should be directed to the General Manager, South Australian Arid Lands Natural Resources Management Board Railway Station Building, PO Box 2227, Port Augusta, SA, 5700 Table of Contents Background ................................................................................................................ 3 The current distribution of alien and native fish species at Edgbaston -

Fishes of Australia's Great Artesian Basin Springs – an Overview
Fishes of Australia’s Great Artesian Basin Springs – An Overview Adam Kerezsy1 Abstract Patterns of fish distribution within Great Artesian Basin springs fall into two distinct categories: the opportunistic colonisation of springs by widespread riverine species following flooding, and long- term habitation – and speciation – within isolated spring complexes by fishes endemic to certain spring complexes. The endemic fishes of Australia’s Great Artesian Basin springs persist in what some would consider the most unlikely fish habitats imaginable. Within predominantly hot and dry landscapes, they inhabit the only reliable wet areas, which are frequently the same temperature as the surrounding plains and as shallow as the body depth of some of the species. There are seven narrow- range fish species endemic to Great Artesian Basin springs: the Dalhousie catfishNeosiluris ( gloveri), Dalhousie hardyhead (Craterocephalus dalhousiensis), red-finned blue-eye Scaturiginichthys( vermeilipinnis), three localised species of gobies (Chlamydogobius gloveri, C. micropterus and C. squami genus) and the Dalhousie mogurnda (Mogurnda thermophila). These species occur at only three locations: Dalhousie in South Australia; and the Pelican Creek and Elizabeth springs complexes, which are both in Queensland. An eighth species, the desert goby (Chlamydogobius eremius) has a wider range across multiple spring complexes in South Australia. All GAB endemic spring species should be considered endangered due to their small ranges and small populations; however, their formal status varies widely between state, national and international legislation and/or lists. Additionally, all fish endemic to GAB springs are threatened by a broad suite of factors that endanger inland aquatic ecosystems, such as water extraction, pollution, and the possibility that alien or unwanted species may become established.