Chalcogen Bonds in Complexes of SOXY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Dependence of the Sio Bond Length on Structural Parameters in Coesite, the Silica Polymorphs, and the Clathrasils
American Mineralogist, Volume 75, pages 748-754, 1990 The dependence of the SiO bond length on structural parameters in coesite, the silica polymorphs, and the clathrasils M. B. BOISEN, JR. Department of Mathematics, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, U.S.A. G. V. GIBBS, R. T. DOWNS Department of Geological Sciences, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, U.S.A. PHILIPPE D' ARCO Laboratoire de Geologie, Ecole Normale Superieure, 75230 Paris Cedex OS, France ABSTRACT Stepwise multiple regression analyses of the apparent R(SiO) bond lengths were com- pleted for coesite and for the silica polymorphs together with the clathrasils as a function of the variables};(O), P (pressure), f,(Si), B(O), and B(Si). The analysis of 94 bond-length data recorded for coesite at a variety of pressures and temperatures indicates that all five of these variables make a significant contribution to the regression sum of squares with an R2 value of 0.84. A similar analysis of 245 R(SiO) data recorded for silica polymorphs and clathrasils indicate that only three of the variables (B(O), };(O), and P) make a signif- icant contribution to the regression with an R2 value of 0.90. The distribution of B(O) shows a wide range of values from 0.25 to 10.0 A2 with nearly 80% of the observations clustered between 0.25 and 3.0 A2 and the remaining values spread uniformly between 4.5 and 10.0 A2. A regression analysis carried out for these two populations separately indicates, for the data set with B(O) values less than three, that };(O) B(O), P, and };(Si) are all significant with an R2 value of 0.62. -

Noncovalent Bonds Through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons
molecules Review Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons Wiktor Zierkiewicz 1,* , Mariusz Michalczyk 1,* and Steve Scheiner 2 1 Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeze˙ Wyspia´nskiego27, 50-370 Wrocław, Poland 2 Department of Chemistry and Biochemistry, Utah State University Logan, Logan, UT 84322-0300, USA; [email protected] * Correspondence: [email protected] (W.Z.); [email protected] (M.M.) Abstract: Over the last years, scientific interest in noncovalent interactions based on the presence of electron-depleted regions called σ-holes or π-holes has markedly accelerated. Their high directionality and strength, comparable to hydrogen bonds, has been documented in many fields of modern chemistry. The current review gathers and digests recent results concerning these bonds, with a focus on those systems where both σ and π-holes are present on the same molecule. The underlying principles guiding the bonding in both sorts of interactions are discussed, and the trends that emerge from recent work offer a guide as to how one might design systems that allow multiple noncovalent bonds to occur simultaneously, or that prefer one bond type over another. Keywords: molecular electrostatic potential; halogen bond; pnicogen bond; tetrel bond; chalcogen bond; cooperativity Citation: Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Noncovalent Bonds through Sigma and Pi-Hole Located on the Same 1. Introduction Molecule. Guiding Principles and The concept of the σ-hole, introduced to a wide audience in 2005 at a conference in Comparisons. Molecules 2021, 26, Prague by Tim Clark [1], influenced a way of thinking about noncovalent interactions 1740. -

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

The Nafure of Silicon-Oxygen Bonds
AmericanMineralogist, Volume 65, pages 321-323, 1980 The nafure of silicon-oxygenbonds LtNus PeurrNc Linus Pauling Institute of Scienceand Medicine 2700 Sand Hill Road, Menlo Park, California 94025 Abstract Donnay and Donnay (1978)have statedthat an electron-densitydetermination carried out on low-quartz by R. F. Stewart(personal communication), which assignscharges -0.5 to the oxygen atoms and +1.0 to the silicon atoms, showsthe silicon-oxygenbond to be only 25 percentionic, and hencethat "the 50/50 descriptionofthe past fifty years,which was based on the electronegativity difference between Si and O, is incorrect." This conclusion, however, ignoresthe evidencethat each silicon-oxygenbond has about 55 percentdouble-bond char- acter,the covalenceof silicon being 6.2,with transferof 2.2 valenc,eelectrons from oxygen to silicon. Stewart'svalue of charge*1.0 on silicon then leads to 52 percentionic characterof the bonds, in excellentagreement with the value 5l percent given by the electronegativity scale. Donnay and Donnay (1978)have recently referred each of the four surrounding oxygen atoms,this ob- to an "absolute" electron-densitydetermination car- servationleads to the conclusionthat the amount of ried out on low-quartz by R. F. Stewart, and have ionic character in the silicon-oxygsa 5ingle bond is statedthat "[t showsthe Si-O bond to be 75 percent 25 percent. It is, however,not justified to make this covalent and only 25 percent ionic; the 50/50 de- assumption. scription of the past fifty years,which was basedon In the first edition of my book (1939)I pointed out the electronegativitydifference between Si and O, is that the observedSi-O bond length in silicates,given incorrect." as 1.604, is about 0.20A less than the sum of the I formulated the electronegativityscale in 1932, single-bond radii of the two atoms (p. -

The Hydrogen Bond: a Hundred Years and Counting
J. Indian Inst. Sci. A Multidisciplinary Reviews Journal ISSN: 0970-4140 Coden-JIISAD © Indian Institute of Science 2019. The Hydrogen Bond: A Hundred Years and Counting Steve Scheiner* REVIEW REVIEW ARTICLE Abstract | Since its original inception, a great deal has been learned about the nature, properties, and applications of the H-bond. This review summarizes some of the unexpected paths that inquiry into this phenom- enon has taken researchers. The transfer of the bridging proton from one molecule to another can occur not only in the ground electronic state, but also in various excited states. Study of the latter process has devel- oped insights into the relationships between the nature of the state, the strength of the H-bond, and the height of the transfer barrier. The enormous broadening of the range of atoms that can act as both pro- ton donor and acceptor has led to the concept of the CH O HB, whose ··· properties are of immense importance in biomolecular structure and function. The idea that the central bridging proton can be replaced by any of various electronegative atoms has fostered the rapidly growing exploration of related noncovalent bonds that include halogen, chalco- gen, pnicogen, and tetrel bonds. Keywords: Halogen bond, Chalcogen bond, Pnicogen bond, Tetrel bond, Excited-state proton transfer, CH O H-bond ··· 1 Introduction charge on the proton. The latter can thus attract Over the course of its century of study following the partial negative charge of an approaching its earliest conceptual formulation1,2, the hydro- nucleophile D. Another important factor resides gen bond (HB) has surrendered many of the in the perturbation of the electronic structure mysteries of its source of stability and its myriad of the two species as they approach one another. -

Atomic Structures of the M Olecular Components in DNA and RNA Based on Bond Lengths As Sums of Atomic Radii
1 Atomic St ructures of the M olecular Components in DNA and RNA based on Bond L engths as Sums of Atomic Radii Raji Heyrovská (Institute of Biophysics, Academy of Sciences of the Czech Republic) E-mail: [email protected] Abstract The author’s interpretation in recent years of bond lengths as sums of the relevant atomic/ionic radii has been extended here to the bonds in the skeletal structures of adenine, guanine, thymine, cytosine, uracil, ribose, deoxyribose and phosphoric acid. On examining the bond length data in the literature, it has been found that the averages of the bond lengths are close to the sums of the corresponding atomic covalent radii of carbon (tetravalent), nitrogen (trivalent), oxygen (divalent), hydrogen (monovalent) and phosphorus (pentavalent). Thus, the conventional molecular structures have been resolved here (for the first time) into probable atomic structures. 1. Introduction The interpretation of the bond lengths in the molecules constituting DNA and RNA have been of interest ever since the discovery [1] of the molecular structure of nucleic acids. This work was motivated by the earlier findings [2a,b] that the inter-atomic distance between any two atoms or ions is, in general, the sum of the radii of the atoms (and or ions) constituting the chemical bond, whether the bond is partially or fully ionic or covalent. It 2 was shown in [3] that the lengths of the hydrogen bonds in the Watson- Crick [1] base pairs (A, T and C, G) of nucleic acids as well as in many other inorganic and biochemical compounds are sums of the ionic or covalent radii of the hydrogen donor and acceptor atoms or ions and of the transient proton involved in the hydrogen bonding. -

Chemical Physics 524 (2019) 55–62
Chemical Physics 524 (2019) 55–62 Contents lists available at ScienceDirect Chemical Physics journal homepage: www.elsevier.com/locate/chemphys Structures of clusters surrounding ions stabilized by hydrogen, halogen, T chalcogen, and pnicogen bonds ⁎ ⁎ Steve Scheinera, , Mariusz Michalczykb, Wiktor Zierkiewiczb, a Department of Chemistry and Biochemistry, Utah State University Logan, Utah 84322-0300, United States b Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeże Wyspiańskiego 27, 50-370 Wrocław, Poland ARTICLE INFO ABSTRACT Keywords: Four H-binding HCl and HF molecules position themselves at the vertices of a tetrahedron when surrounding a Tetrahedral central Cl−. Halogen bonding BrF and ClF form a slightly distorted tetrahedron, a tendency that is amplified for QTAIM ClCN which forms a trigonal pyramid. Chalcogen bonding SF2, SeF2, SeFMe, and SeCSe occupy one hemisphere NBO of the central ion, leaving the other hemisphere empty. This pattern is repeated for pnicogen bonding PF3, SeF3 Cl− and AsCF. The clustering of solvent molecules on one side of the Cl− is attributed to weak attractive interactions Na+ between them, including chalcogen, pnicogen, halogen, and hydrogen bonds. Binding energies of four solvent molecules around a central Na+ are considerably reduced relative to chloride, and the geometries are different, with no empty hemisphere. The driving force maximizes the number of electronegative (F or O) atoms close to the Na+, and the presence of noncovalent bonds between solvent molecules. 1. Introduction particular, the S, Se, etc group can engage in chalcogen bonds (YBs) [19–25], and the same is true of the P, As family which forms pnicogen The hydrogen bond (HB) is arguably the most important and in- bonds (ZBs) [26–34]. -

Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc
Metal-Metal (MM) Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc Richard H. Duncan Lyngdoh*,a, Henry F. Schaefer III*,b and R. Bruce King*,b a Department of Chemistry, North-Eastern Hill University, Shillong 793022, India B Centre for Computational Quantum Chemistry, University of Georgia, Athens GA 30602 ABSTRACT: This survey of metal-metal (MM) bond distances in binuclear complexes of the first row 3d-block elements reviews experimental and computational research on a wide range of such systems. The metals surveyed are titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc, representing the only comprehensive presentation of such results to date. Factors impacting MM bond lengths that are discussed here include (a) n+ the formal MM bond order, (b) size of the metal ion present in the bimetallic core (M2) , (c) the metal oxidation state, (d) effects of ligand basicity, coordination mode and number, and (e) steric effects of bulky ligands. Correlations between experimental and computational findings are examined wherever possible, often yielding good agreement for MM bond lengths. The formal bond order provides a key basis for assessing experimental and computationally derived MM bond lengths. The effects of change in the metal upon MM bond length ranges in binuclear complexes suggest trends for single, double, triple, and quadruple MM bonds which are related to the available information on metal atomic radii. It emerges that while specific factors for a limited range of complexes are found to have their expected impact in many cases, the assessment of the net effect of these factors is challenging. -

Quantitative Analysis of Hydrogen and Chalcogen Bonds in Two Pyrimidine
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Quantitative analysis of hydrogen and chalcogen bonds in two pyrimidine-5-carbonitrile derivatives, potential DHFR inhibitors: an integrated crystallographic and theoretical study Al-Wahaibi, Lamya H ; Chakraborty, Kushumita ; Al-Shaalan, Nora H ; Syed Majeed, Mohamed Yehya Annavi ; Blacque, Olivier ; Al-Mutairi, Aamal A ; El-Emam, Ali A ; Percino, M Judith ; Thamotharan, Subbiah Abstract: Two potential bioactive pyrimidine-5-carbonitrile derivatives have been synthesized and char- acterized by spectroscopic techniques (1H and 13C-NMR) and the three dimensional structures were elucidated by single crystal X-ray diffraction at low temperature (160 K). In both structures, the molec- ular conformation is locked by an intramolecular C–HC interaction involving the cyano and CH of the thiophene and phenyl rings. The intermolecular interactions were analyzed in a qualitative man- ner based on the Hirshfeld surface and 2D-fingerprint plots. The results suggest that the phenyl and thiophene moieties have an effect on the crystal packing. For instance, the chalcogen bonds areonly preferred in the thiophene derivative. However, both structures uses a common N–HO hydrogen bond motif. Moreover, the structures of 1 and 2 display 1D isostructurality and molecular chains stabilize by intermolecular N–HO and N–HN hydrogen bonds. The nature and extent of different non-covalent interactions were further characterized by the topological parameters derived from the quantum the- ory of atoms-in-molecules approach. This analysis indicates that apart from N–HO hydrogen bonds, other non-covalent interactions are closed-shell in nature. -
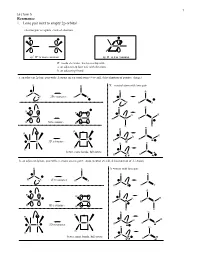
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚.