Steidle Building COVID RTRP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Instant Messaging Market, 2009-2013 Executive Summary
THE RADICATI GROUP, INC. A TECHNOLOGY MARKET RESEARCH FIRM 1900 EMBARCADERO ROAD, SUITE 206. • PALO ALTO, CA 94303 TEL. 650 322-8059 • FAX 650 322-8061 Instant Messaging Market, 2009-2013 Editor: Sara Radicati, Ph.D; Principal Analyst: Todd Yamasaki SCOPE This study examines the market for Instant Messaging (IM) solutions from 2009 to 2013. It provides extensive data regarding current installed base, broken out by region, business size, and other variables, including four year forecasts. This report also examines IM solution features, business strategies, plus product strengths and weaknesses. All market numbers, such as market size, forecasts, installed base, and any financial information presented in this study represent worldwide figures, unless otherwise indicated. All pricing numbers are expressed in $USD. METHODOLOGY The information and analysis in this report is based on primary research conducted by The Radicati Group, Inc. It consists of information collected from vendors, and users within global corporations via interviews and surveys. Secondary research sources have also been used, where appropriate, to cross-check the information collected. These include company annual reports and market size information from various market segments of the computer industry. EUROPE: 29E FITZJOHNS AVE • LONDON NW3 5JY • TEL. +44 (0)207 794 4298 • FAX. +44 (0)207 431 9375 e-mail: [email protected] http://www.radicati.com Instant Messaging Market, 2009-2013 – Executive Summary EXECUTIVE SUMMARY EXECUTIVE SUMMARY This study looks at the Instant Messaging market as comprising four different market segments: o Public IM networks – This segment includes free IM services which primarily target consumers, but are also prevalent amongst business users. -

Survey of Instant Messaging Applications Encryption Methods
Avrupa Bilim ve Teknoloji Dergisi European Journal of Science and Technology Cilt. 2, No. 4, S. 112-117, Haziran 2015 Vol. 2, No. 4, pp. 112-117, June 2015 © Telif hakkı EJOSAT’a aittir Copyright © 2014 EJOSAT Araştırma Makalesi www.ejosat.com ISSN:2148-2683 Research Article Survey of Instant Messaging Applications Encryption Methods Abdullah Talha Kabakus1*, Resul Kara2 1 Abant Izzet Baysal University, IT Center, 14280, Bolu, Turkey 2 Duzce University, Faculty of Engineering, Department of Computer Engineering, 81620, Duzce, Turkey (First received 15 February 2015 and in final form 29 May 2015) Abstract Instant messaging applications has already taken the place of traditional Short Messaging Service (SMS) and Multimedia Messaging Service (MMS) due to their popularity and usage easement they provide. Users of instant messaging applications are able to send both text and audio messages, different types of attachments such as photos, videos, and contact information to their contacts in real time. Because of instant messaging applications use internet instead of Short Message Service Technical Realization (GSM), they are free to use and they only require internet connection which is the most common way of communication today. The critical point here is providing privacy of these messages in order to prevent any vulnerable points for hackers and cyber criminals. According to the latest research by PricewaterhouseCoopers, percentage of global cyber attacks is increased to 48% with 42.8 million detected incidents. Another report that is published by security company Postini indicates that 90% of instant messaging targeted threats are highly destructive worms. In this study, instant messaging applications encryption methods are comparatively presented. -

Guess Who's Texting You? Evaluating the Security of Smartphone
Guess Who’s Texting You? Evaluating the Security of Smartphone Messaging Applications Sebastian Schrittwieser, Peter Fruhwirt,¨ Peter Kieseberg, Manuel Leithner, Martin Mulazzani, Markus Huber, Edgar Weippl SBA Research gGmbH Vienna, Austria (1stletterfirstname)(lastname)@sba-research.org Abstract been the subject of an ample amount of past research. The common advantages of the tools we examined lie in In recent months a new generation of mobile messag- very simple and fast setup routines combined with the possi- ing and VoIP applications for smartphones was introduced. bility to incorporate existing on-device address books. Ad- These services offer free calls and text messages to other ditionally these services offer communication free of charge subscribers, providing an Internet-based alternative to the and thus pose a low entry barrier to potential customers. traditional communication methods managed by cellular However, we find that the very design of most of these mes- network carriers such as SMS, MMS and voice calls. While saging systems thwarts their security measures, leading to user numbers are estimated in the millions, very little atten- issues such as the possibility for communication without tion has so far been paid to the security measures (or lack proper sender authentication. thereof) implemented by these providers. The main contribution of our paper is an evaluation of the In this paper we analyze nine popular mobile messaging security of mobile messaging applications with the afore- and VoIP applications and evaluate their security models mentioned properties and the possibilities of abuse in real- with a focus on authentication mechanisms. We find that a world scenarios. -

Case No COMP/M.6281 - MICROSOFT/ SKYPE
EN Case No COMP/M.6281 - MICROSOFT/ SKYPE Only the English text is available and authentic. REGULATION (EC) No 139/2004 MERGER PROCEDURE Article 6(1)(b) NON-OPPOSITION Date: 07/10/2011 In electronic form on the EUR-Lex website under document number 32011M6281 Office for Publications of the European Union L-2985 Luxembourg EUROPEAN COMMISSION Brussels, 07/10/2011 C(2011)7279 In the published version of this decision, some information has been omitted pursuant to Article MERGER PROCEDURE 17(2) of Council Regulation (EC) No 139/2004 concerning non-disclosure of business secrets and other confidential information. The omissions are shown thus […]. Where possible the information omitted has been replaced by ranges of figures or a general description. PUBLIC VERSION To the notifying party: Dear Sir/Madam, Subject: Case No COMP/M.6281 - Microsoft/ Skype Commission decision pursuant to Article 6(1)(b) of Council Regulation No 139/20041 1. On 02.09.2011, the European Commission received notification of a proposed concentration pursuant to Article 4 of the Merger Regulation by which the undertaking Microsoft Corporation, USA (hereinafter "Microsoft"), acquires within the meaning of Article 3(1)(b) of the Merger Regulation control of the whole of the undertaking Skype Global S.a.r.l, Luxembourg (hereinafter "Skype"), by way of purchase of shares2. Microsoft and Skype are designated hereinafter as "parties to the notified operation" or "the parties". I. THE PARTIES 2. Microsoft is active in the design, development and supply of computer software and the supply of related services. The transaction concerns Microsoft's communication services, in particular the services offered under the brands "Windows Live Messenger" (hereinafter "WLM") for consumers and "Lync" for enterprises. -

Secure Browser-Based Instant Messaging
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2012-09-22 Secure Browser-Based Instant Messaging Christopher Douglas Robison Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Computer Sciences Commons BYU ScholarsArchive Citation Robison, Christopher Douglas, "Secure Browser-Based Instant Messaging" (2012). Theses and Dissertations. 3372. https://scholarsarchive.byu.edu/etd/3372 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Secure Browser-Based Instant Messaging Christopher D. Robison A thesis submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Master of Science Kent E. Seamons, Chair Daniel M. A. Zappala Sean C. Warnick Department of Computer Science Brigham Young University December 2012 Copyright c 2012 Christopher D. Robison All Rights Reserved ABSTRACT Secure Browser-Based Instant Messaging Christopher D. Robison Department of Computer Science, BYU Master of Science Instant messaging is a popular form of communication over the Internet. Statistics show that instant messaging has overtaken email in popularity. Traditionally, instant messaging has consisted of a desktop client communicating with other clients via an instant messaging service provider. However, instant messaging solutions are starting to become available in the web browser{services like Google Talk, Live Messenger and Facebook. Despite the work done by researchers to secure instant messaging networks, little work has been done to secure instant messaging in the browser. -

RELEASE NOTES UFED PHYSICAL ANALYZER, Version 5.0 | March 2016 UFED LOGICAL ANALYZER
NOW SUPPORTING 19,203 DEVICE PROFILES +1,528 APP VERSIONS UFED TOUCH, UFED 4PC, RELEASE NOTES UFED PHYSICAL ANALYZER, Version 5.0 | March 2016 UFED LOGICAL ANALYZER COMMON/KNOWN HIGHLIGHTS System Images IMAGE FILTER ◼ Temporary root (ADB) solution for selected Android Focus on the relevant media files and devices running OS 4.3-5.1.1 – this capability enables file get to the evidence you need fast system and physical extraction methods and decoding from devices running OS 4.3-5.1.1 32-bit with ADB enabled. In addition, this capability enables extraction of apps data for logical extraction. This version EXTRACT DATA FROM BLOCKED APPS adds this capability for 110 devices and many more will First in the Industry – Access blocked application data with file be added in coming releases. system extraction ◼ Enhanced physical extraction while bypassing lock of 27 Samsung Android devices with APQ8084 chipset (Snapdragon 805), including Samsung Galaxy Note 4, Note Edge, and Note 4 Duos. This chipset was previously supported with UFED, but due to operating system EXCLUSIVE: UNIFY MULTIPLE EXTRACTIONS changes, this capability was temporarily unavailable. In the world of devices, operating system changes Merge multiple extractions in single unified report for more frequently, and thus, influence our support abilities. efficient investigations As our ongoing effort to continue to provide our customers with technological breakthroughs, Cellebrite Logical 10K items developed a new method to overcome this barrier. Physical 20K items 22K items ◼ File system and logical extraction and decoding support for iPhone SE Samsung Galaxy S7 and LG G5 devices. File System 15K items ◼ Physical extraction and decoding support for a new family of TomTom devices (including Go 1000 Point Trading, 4CQ01 Go 2505 Mm, 4CT50, 4CR52 Go Live 1015 and 4CS03 Go 2405). -

Ebuddy Software for Nokia 6300
Ebuddy software for nokia 6300 click here to download Chat on MSN, Facebook, Yahoo!, GTalk (Orkut), AIM, MySpace and ICQ. Get the most popular free chat app on your phone, with more than million. eBuddy Messenger - Chat on Facebook, MSN, Yahoo!, Orkut (Google Talk), AIM, ICQ & MySpace. Get the most popular free IM app on your. eBuddy - EBuddy is samsung s for Nokia Free Download. Max99 is a global community software that lets you keep in touch. Get free downloadable EBuddy Nokia Java Apps for your mobile device. Free mobile Latest version of eBuddy Mobile Messenger with several bug fixes . Facebook chat messenger for mobile nokia All Manuals online it Chat flirt Apps Stay mais 1 install 1 on Software any Nokia EBuddy. Now you can Chat with your Friends on the Biggest Social Network In the World on your Mobile for Nokia Free App Download. Java ebuddy mobile nokia. On nokia ,sms chat nokia free,nokia c facebook chat software. Ebuddy facebook messenger downloader mobile nokia I use a Nokia that I recently downloaded Ebuddy I buy, I also of CI, but when I run the application Ebuddy, an error message and tell. Hi i have a nokia and i have recently downloaded and installed Ebuddy mobile messenger version but when i lauch it, my mobile. ebuddy messenger for Download, ebuddy messenger for, ebuddy messenger for free download, free mp3 music pic monkey photo nokia space. Facebook Messenger latest version: Official Facebook chat app for Nokia S Facebook Messenger (Nokia Series 40) eBuddy Mobile Messenger. Send free text messages with WhatsApp on your Nokia phone . -

Exinda Applications List
Application List Exinda ExOS Version 6.4 © 2014 Exinda Networks, Inc. 2 Copyright © 2014 Exinda Networks, Inc. All rights reserved. No parts of this work may be reproduced in any form or by any means - graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems - without the written permission of the publisher. Products that are referred to in this document may be either trademarks and/or registered trademarks of the respective owners. The publisher and the author make no claim to these trademarks. While every precaution has been taken in the preparation of this document, the publisher and the author assume no responsibility for errors or omissions, or for damages resulting from the use of information contained in this document or from the use of programs and source code that may accompany it. In no event shall the publisher and the author be liable for any loss of profit or any other commercial damage caused or alleged to have been caused directly or indirectly by this document. Document Built on Tuesday, October 14, 2014 at 5:10 PM Documentation conventions n bold - Interface element such as buttons or menus. For example: Select the Enable checkbox. n italics - Reference to other documents. For example: Refer to the Exinda Application List. n > - Separates navigation elements. For example: Select File > Save. n monospace text - Command line text. n <variable> - Command line arguments. n [x] - An optional CLI keyword or argument. n {x} - A required CLI element. n | - Separates choices within an optional or required element. © 2014 Exinda Networks, Inc. -

Strategic Performance Measurement for Ebuddy
Strategic performance measurement for eBuddy May 2007 Graduation thesis of: V.J.M. Hoogsteder Student number 9901795 Business Information Technology Faculty of Electrical Engineering, Mathematics and Computer Science (EEMCS) University of Twente [email protected] +31 (0) 6 16 376 233 On behalf of: eBuddy Keizersgracht 585 1017 DR Amsterdam +31 (0) 20 514 1430 Under supervision of: P. Bliek (University of Twente, School of Management and Governance, NIKOS group) L. Bodenstaff (University of Twente, faculty of EEMCS, Information Systems group) J.J. Rueb (CEO eBuddy) 1 Management summary Motivation eBuddy management wants to define and implement strategic performance measurement to improve strategic decision making by reducing the necessary time to support decisions and by gaining a solid and complete view on eBuddy’s strategic performance. Recommendations 1) Assess strategic performance with the strategic performance measures identified in eBuddy’s balanced scorecard, as defined in this research; 2) Assign a responsible employee for monthly reporting of the balanced scorecard; 3) Update the balanced scorecard based on changes in strategic objectives; 4) Define desired employee skills and measure these in the balanced scorecard; 5) Develop an information system to present the defined strategic performance measures; 6) Involve business unit directors in strategic performance assessment; 7) Use the balanced scorecard to facilitate problem solving and action planning. Argumentation 1) The eBuddy balanced scorecard is defined according to Kaplan & Norton’s model (Kaplan & Norton 1996). This model provides a comprehensive set of guidelines for definition and implementation of strategic performance measurement and is supported by extensive literature attention. The steps taken towards the definition and implementation of eBuddy’s balanced scorecard were based on the roadmap identified by Assiri et al. -
3000 Applications
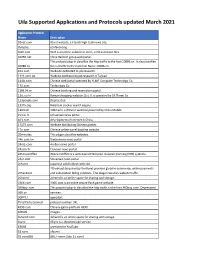
Uila Supported Applications and Protocols updated March 2021 Application Protocol Name Description 01net.com 05001net plus website, is a Japanese a French embedded high-tech smartphonenews site. application dedicated to audio- 050 plus conferencing. 0zz0.com 0zz0 is an online solution to store, send and share files 10050.net China Railcom group web portal. This protocol plug-in classifies the http traffic to the host 10086.cn. It also classifies 10086.cn the ssl traffic to the Common Name 10086.cn. 104.com Web site dedicated to job research. 1111.com.tw Website dedicated to job research in Taiwan. 114la.com Chinese cloudweb portal storing operated system byof theYLMF 115 Computer website. TechnologyIt is operated Co. by YLMF Computer 115.com Technology Co. 118114.cn Chinese booking and reservation portal. 11st.co.kr ThisKorean protocol shopping plug-in website classifies 11st. the It ishttp operated traffic toby the SK hostPlanet 123people.com. Co. 123people.com Deprecated. 1337x.org Bittorrent tracker search engine 139mail 139mail is a chinese webmail powered by China Mobile. 15min.lt ChineseLithuanian web news portal portal 163. It is operated by NetEase, a company which pioneered the 163.com development of Internet in China. 17173.com Website distributing Chinese games. 17u.com 20Chinese minutes online is a travelfree, daily booking newspaper website. available in France, Spain and Switzerland. 20minutes This plugin classifies websites. 24h.com.vn Vietnamese news portal 24ora.com Aruban news portal 24sata.hr Croatian news portal 24SevenOffice 24SevenOffice is a web-based Enterprise resource planning (ERP) systems. 24ur.com Slovenian news portal 2ch.net Japanese adult videos web site 2Checkout (acquired by Verifone) provides global e-commerce, online payments 2Checkout and subscription billing solutions. -

Evaluating the Security of Smartphone Messaging Applications
TelcoSecDay @ Troopers � 3/20/12 � Heidelberg, Germany Guess Who’s Texting You? Evaluating the Security of Smartphone Messaging Applications Sebastian Schrittwieser SBA Research, Vienna, Austria Source: path.com address book-gate android.permission.READ_CONTACTS android.permission.READ_CALENDAR android.permission.INTERNET Analyzing network traffic of smartphones • Data flow analysis • Security evaluation • Example: Smartphone Messengers Smartphone Messaging • Aim at replacing traditional text messaging (SMS) and GSM/CDMA/3G calls • Free phone calls and text messages over the Internet • Novel authentication concept • Phone number used as single authenticating identifier Internet Telecom infrastructure Motivation Traditional SMS/talk Messenger/VoIP Apps Protocol proprietary HTTP(S), XMPP cryptographically sound application depended, much Security authentication weaker authentication (SIM card) (phone number, IMSI, UDID) Users’ SMS/talk perception Evaluation Authentication Mechanism and Account Hijacking Sender ID Spoofing / Message Manipulation Unrequested SMS / phone calls User Enumeration Modifying Status Messages Experimental Setup • Samsung Nexus S running Android 2.3.3 and Apple iPhone 4 running iOS 4.3.3 • SSL proxy to read encrypted HTTPS traffic Phone SSL-Interception Server • Used to understand the protocol, not for the actual attack (i.e., MITM between victim and server)! Certificates? WhatsApp eBuddy XMS WowTalk Viber HeyTell Forfone Voypi Tango EasyTalk WhatsApp Paper: Guess who’s texting you? Evaluating the Security of Smartphone Messaging Applications Schrittwieser, S., Frühwirt, P., Kieseberg, P., Leithner, M., Mulazzani, M., Huber, M., Weippl, E., NDSS 2012 WhatsApp • Instant Messaging • Status messages • 23+ million users worldwide (estimation) • > 1 billion messages per day • Clients available for Android, iOS, Symbian and Blackberry Authentication in WhatsApp 1. (HTTPS): Phone number 2. (SMS): Code SMS Proxy 3. -

Online Identifiers in Everyday Life
© 2010 by Benjamin M. Gross. All rights reserved. ONLINE IDENTIFIERS IN EVERYDAY LIFE BY BENJAMIN M. GROSS DISSERTATION Submied in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Library and Information Science in the Graduate College of the University of Illinois at Urbana-Champaign, 2010 Urbana, Illinois Doctoral Commiee: Associate Professor Michael Twidale, Chair Professor Geof Bowker, University of Pisburgh Professor Chip Bruce Associate Professor Ann Bishop Abstract Identifiers are an essential component of online communication. Email addresses and instant messenger usernames are two of the most common online identi- fiers. is dissertation focuses on the ways that social, technical and policy fac- tors affect individual’s behavior with online identifiers. Research for this dissertation was completed in two parts, an interview-based study drawn from two populations and an examination of the infrastructure for managing identifiers in two large consumer services. e exploratory study ex- amines how individuals use online identifiers to segment and integrate aspects of their lives. e first population is drawn from employees of a financial ser- vice firm with substantial constraints on communication in the workplace. e second population is drawn from a design firm with minimal constraints on com- munication. e two populations provide the opportunity to explore the social, technical, and policy issues that arise from diverse communication needs, uses, strategies, and technologies. e examination of systems focuses on the infras- tructure that Google and Yahoo! provide for individuals to manage their iden- tifiers across multiple services, and the risks and benefits of employing single sign-on systems.