Evolutionary Patterns and Processes in the Genus Potentilla L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Unique Raised Bog at Urbana, Ohio.*
A UNIQUE RAISED BOG AT URBANA, OHIO.* ROBERT B. GORDON, Ohio State University. Located just north of the Champaign County Fair Grounds at Urbana, Ohio, is a unique dome-shaped bog, covered with shrubby vegetation for the most part, in which the center is raised at least ten feet above the margins. An old road crosses the bog. I have been told that it was once the main thorofare from Urbana to Columbus. Horses and wagons passed over it, I suppose, the drivers never realizing that a mat of fibrous roots less than one foot thick was all that held them over a body of water twelve feet in depth. Raised bogs, called "high moors" and "Hochmoore" in foreign literature, have long been known throughout Europe. N. S. Shaler is credited by Nichols with being the first to call attention to these peculiar swamps in North America, in 1888-89. Those which Shaler observed were "mostly limited to the eastern portion of Maine, near the shores of the Bay of Fundy," but some of lesser magnitude were reported for New Hampshire, northern Michigan, and Minnesota. Similar bogs, with centers about 13 feet above their margins, have been reported in the province of New Brunswick by Ganong (1897). Nichols (1919) described bogs of this type encountered in Maine, in which the elevation of the center above the margin varied from 2 or 3 feet to as high as 18 feet (e. g., Denbo Heath, covering several square miles in area). He asserts: "(1) that in the state of Maine raised bogs, in so far as they constitute a distinctive swamp type, are virtually restricted to the proximity of the seacoast; and (2) that in other portions of New England and of the eastern United States this type of bog is practically absent, although in occasional swamps it is possible to detect a slight elevation of the surface above the level of permanent ground water." Warming (1909) has summarized concisely the characteristic features of "Hochmoore." They owe their development to the growth of sphagnum mosses which absorb water that falls in the form of rain or snow. -

Plateau Mountain Plant List
Plateau ALBERTA WILDERNESS Mountain – Plant list ASSOCIATION Plants of Plateau Mountain Ecological Reserve (Plateau Mountain Ecological Reserve Management Plan – Alberta Environment) Alpine Anemone Anemone drummondii Drummond's Rock Cress Alpine Arnica Arnica angustifolia Drummond's Rush Alpine Bistort Polygonum viviparum Dwarf Birch Betula glandulosa Alpine Blue Grass Poa alpina Dwarf Bitter root Alpine Everlasting Antennaria sp. Dwarf Hawk's Beard Alpine Fleabane Erigeron pallens Dwarf Sawwort Saussurea nuda Alpine Forget-me not Myosotis alpestris Dwarf saw-wort Saussurea nuda Alpine Goldenrod Solidago multiradianta Dwarf Scouring-rush Equisetum scirpoides Alpine Hawkweed Early Blue Grass Alpine Milk-vetch Astragalus alpinus Early Blue Violet Viola adunca Alpine mouse-eared chickweed Cerastium beeringianum Early Cinquefoil Potertilla concinna Alpine Speedwell Veronica alpina Elephant's head Pedicularis groenlandica Alpine speedwell Veronica alpina Elephant's-head Lousewort Pedicualaris groenlandica Alpine Timothy Elgelmann Spruce Picea engelmanii American Vetch Entire-leaved Groundsel Androsace Androsace chamaejasme Everlasting Antennaria luzuloides Arctic Aster False Dandelion Agoseris aurantiaca Arctic Blue Glass Felwort Gentianela amarella Arctic Butterweed Few-flowered Milk-vetch Astragalus sp. Balsam Groundsel Few-seeded Whitlow-grass Draba oligosperma Balsam Poplar Populus balsamifera Field Chickweed Stellaria sp. Barratt's Willow Fireweed Locoweed Oxytropis sp. Bearberry Arctostaphylos uva-ursi Flame-colored Lousewort Pedicularis -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Comparative Analysis of the Complete Chloroplast Genome Sequences of Three Closely Related East-Asian Wild Roses (Rosa Sect
G C A T T A C G G C A T genes Article Comparative Analysis of the Complete Chloroplast Genome Sequences of Three Closely Related East-Asian Wild Roses (Rosa sect. Synstylae; Rosaceae) Ji-Hyeon Jeon and Seung-Chul Kim * Department of Biological Sciences, Sungkyunkwan University, Suwon 16419, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-31-299-4499 Received: 4 November 2018; Accepted: 27 December 2018; Published: 3 January 2019 Abstract: Species belonging to Rosa section Synstylae (Rosaceae) are mainly distributed in East Asia, and represent recently diverged lineages within the genus. Over decades, inferring phylogenetic relationships within section Synstylae have been exceptional challenges, due to short branch lengths and low support values. Of approximately 36 species in the section Synstylae, Rosa multiflora, Rosa luciae and Rosa maximowicziana are widely distributed in the Sino-Japanese floristic region. In this study, we assembled chloroplast genomes of these three species to compare the genomic features within section Synstylae, and to compare with other infrageneric groups. We found that three Rosa sect. Synstylae species had lost infA genes with pseudogenization, and they were almost identical to each other. Two protein-coding gene regions (ndhF and ycf1) and five non-coding regions (5’matK-trnK, psbI-trnS-trnG, rps16-trnG, rpoB-trnC, and rps4-trnT) were identified as being highly informative markers. Within three section Synstylae chloroplast genomes, 85 simple sequence repeat (SSR) motifs were detected, of which at least 13 motifs were identified to be effective markers. The phylogenetic relationships of R. multiflora, R. luciae and R. maximowicziana could not be resolved, even with chloroplast genome-wide data. -

Draft Plant Propagation Protocol
Plant Propagation Protocol for Dasiphora fruticosa ESRM 412 – Native Plant Production Protocol URL: https://courses.washington.edu/esrm412/protocols/DAFR.pdf Figure 1: Distribution in North America7 Figure 2: Distribution in Washington State7 TAXONOMY7,8 Plant Family Scientific Rosaceae Name Common Name Rose family Species Scientific Name Scientific Dasiphora fruticosa (L.) Rydb. Name Varieties Sub-species Dasiphora fruticosa (L.) Rydb. subsp. fruticosa Dasiphora fruticosa (L.) Rydb. ssp. floribunda (Pursh) Kartesz Cultivar Dozens of cultivars have been created for variations in flower shape and color, foliage appearance, and shrub form.9 Common Dasiphora floribunda (Pursh) Raf. Synonym(s) Potentilla fruticosa L. Common Shrubby cinquefoil, bush cinquefoil, golden hardhack, widdy Name(s) Species Code DAFR6 (as per USDA Plants database) GENERAL INFORMATION Geographical North America (Canada and United States), Europe, Asia.9,10 See maps above range for detailed distribution in N. America and Washington State.7 Ecological Mid to high-elevation meadows, rocky slopes, open forest, riparian areas and distribution swamps.3,11 Climate and Temperate and subarctic.9,11 elevation range Local habitat Locally found in Washington counties bordering the Cascades, the Olympic and peninsula, and in the northeast bordering Idaho.7 abundance Commonly associated in the wild with Carex, Penstemon, Elymus, and Potentilla species.3 Plant strategy Colonizer, early seral species.12 type / successional stage Plant Perennial shrub able to withstand harsh environmental conditions, including characteristic cold temperatures, strong wind, full sun, and drought.5,9,11 Can tolerate a wide s pH range.11 Adapted to grow in a range of soil conditions, including variable texture and calcareous soils, but grows best in sandy and loamy soil.5,9 This short slow-growing rounded shrub can grow up to 1m tall and 1m wide with numerous small, narrowly oblong green leaves3,9. -

Pala Park Habitat Assessment
Pala Park Bank Stabilization Project: Geotechnical Exploration TABLE OF CONTENTS SECTION 1.0 COUNTY OF RIVERSIDE ATTACHMENTS Biological Report Summary Report (Attachment E-3) Level of Significance Checklist (Attachment E-4) Biological Resources Map (Attachment E-5) Site Photographs (Attachment E-6) SECTION 2.0 HABITAT ASSESSMENT General Site Information ............................................................................................................... 1 Methods ........................................................................................................................................ 2 Existing Conditions ....................................................................................................................... 4 Special Status Resources ............................................................................................................. 8 Other Issues ................................................................................................................................ 14 Recommendations ...................................................................................................................... 14 References .................................................................................................................................. 16 LIST OF TABLES Page 1 Special Status Plant Species Known to Occur in the Vicinity of the Survey Area ........... 10 2 Chaparral Sand-Verbena Populations Observed in the Survey Area ............................. 12 3 Paniculate Tarplant -

Autumn Willow in Rocky Mountain Region the Black Hills National
United States Department of Agriculture Conservation Assessment Forest Service for the Autumn Willow in Rocky Mountain Region the Black Hills National Black Hills National Forest, South Dakota and Forest Custer, South Dakota Wyoming April 2003 J.Hope Hornbeck, Carolyn Hull Sieg, and Deanna J. Reyher Species Assessment of Autumn willow in the Black Hills National Forest, South Dakota and Wyoming J. Hope Hornbeck, Carolyn Hull Sieg and Deanna J. Reyher J. Hope Hornbeck is a Botanist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. in Environmental Biology (botany emphasis) at The University of Montana and a M.S. in Plant Biology (plant community ecology emphasis) at the University of Minnesota-Twin Cities. Carolyn Hull Sieg is a Research Plant Ecologist with the Rocky Mountain Research Station in Flagstaff, Arizona. She completed a B.S. in Wildlife Biology and M.S. in Range Science from Colorado State University and a Ph.D. in Range and Wildlife Management (fire ecology) at Texas Tech University. Deanna J. Reyher is Ecologist/Soil Scientist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. degree in Agronomy (soil science and crop production emphasis) from the University of Nebraska – Lincoln. EXECUTIVE SUMMARY Autumn willow, Salix serissima (Bailey) Fern., is an obligate wetland shrub that occurs in fens and bogs in the northeastern United States and eastern Canada. Disjunct populations of autumn willow occur in the Black Hills of South Dakota. Only two populations occur on Black Hills National Forest lands: a large population at McIntosh Fen and a small population on Middle Boxelder Creek. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -
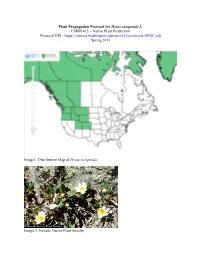
Plant Propagation Protocol for Dryas Octopetala L. ESRM 412 – Native Plant Production Protocol URL
Plant Propagation Protocol for Dryas octopetala L. ESRM 412 – Native Plant Production Protocol URL: https://courses.washington.edu/esrm412/protocols/DROC.pdf Spring 2015 Image1. Distribution Map of Dryas octopetala Image 2. Nevada Native Plant Society TAXONOMY Plant Family [3] Scientific Name Rosaceae Common Name Rose Family Species Scientific Name Scientific Name Dryas octopetala L. [3] Varieties * Dryas octopetala var. angustifolia C.L. Hitchc. [3] Dryas octopetala var. hookeriana (Juz) Hulten Sub-species Dryas octopetala f. argentea (Blytt) Hulten * Dryas octopetala subsp. alaskensis (Porsild) Hulten [3] Cultivar Common Synonym(s) Dradetum octopetalae Keiner Common Name(s) White mountain-avens, Eightpetal mountain-avens, Mountain dryas Species Code (as per USDA Plants DROC database) GENERAL INFORMATION Geographical range Alaska, Washington, Oregon, Colorado Alpine regions in the Pacific Northwest N. Cascades, and Rocky Mountain ranges Ecological distribution Mid-montane to Alpine zone Climate and elevation range Elevation: 3,500 m [6] 100 m and less Climate: sites with low snow cover on calcareous or basic soils [6] Local habitat and abundance Full sun Dry, well-drained, sandy or gravelly soils Spreads rapidly [6] Dominant or co-dominant species within its range [6] Associated species: Dwarf willow Plant strategy type / successional Nitrogen fixer: forms association with Frankia [5] stage Colonizer of barren slopes at high elevations [5] Plant characteristics Forb/herb, Shrub, Subshrub Perrennial Forms mats up to 3 ft. wide and 8 in. tall. [1] 1 cream or white flower at the end of each 2-8 inch leafless flower stalk. [2] Flowers bloom June-July Summer fruits fluffy and feathery Seeds are wind-dispersed [7] Leaves are oval-shaped, leathery with rounded teeth and a white underside. -

CHANGES in the DISTRIBUTION RANGE of POTENTILLA TUCUMANENS/S (ROSACEAE), an ENDANGERED CRYPTIC Speciesi
ISSN 0373-580 X Bol. Soc. Argent. Bot. 36 (1-2): 151 - 157. 2001 CHANGES IN THE DISTRIBUTION RANGE OF POTENTILLA TUCUMANENS/S (ROSACEAE), AN ENDANGERED CRYPTIC SPECIESi MARTA ARIAS23, JUAN DIAZ RICCI4 and ATILIO CASTAGNARO4 Summary: A correct taxonomic identification is essential for the preservation of biodiversity. In this paper we present simple morphological characters for the identification of the new species recently described, Potentilla tucumanensis. The reproductive period of this species occupies about 10% of the last part of its lifecycle, is considered cryptic because in the vegetative stage it has many morphological similarities with other cohabiting related species; it can be easily confused with them and it is reproductively isolated. Our study also revealed that for the last hundred years P. tucumanensis has suffered a dramatic habitat retraction. Possible causes of this area retraction are discussed. If P tucumanensis had not been recognized asa new species, it would have most likely disappeared without being noticed. Key words: Potentilla tucumanensis, Duchesnea indica, Fragaria vesca, endangered species, cryptic species, anthropogenic changes. Resumen: Cambios en el rango de distribución de Potentilla tucumanensis (Rosaceae), una especie críptica en peligro de extinción. Una correcta identificación taxonómica es esencial para la preservación de la biodiversidad. En este trabajo se presentan caracteres morfológicos sencillos para diferenciar la nueva especip, Potentilla tucumanensis recientemente descripta. Esta especie, cuyo período reproductivo ocupa el 10% final de su ciclo de vida, es considerada críptica debido a que puede ser confundida por su similitud en estado vegetativo, con especies con las cuales cohabita y está aislada reproductivamente. Además, se muestra que en los últimos cien años, P. -

Rare Plant Survey of San Juan Public Lands, Colorado
Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Peggy Lyon and Julia Hanson Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 December 2005 Cover: Imperiled (G1 and G2) plants of the San Juan Public Lands, top left to bottom right: Lesquerella pruinosa, Draba graminea, Cryptantha gypsophila, Machaeranthera coloradoensis, Astragalus naturitensis, Physaria pulvinata, Ipomopsis polyantha, Townsendia glabella, Townsendia rothrockii. Executive Summary This survey was a continuation of several years of rare plant survey on San Juan Public Lands. Funding for the project was provided by San Juan National Forest and the San Juan Resource Area of the Bureau of Land Management. Previous rare plant surveys on San Juan Public Lands by CNHP were conducted in conjunction with county wide surveys of La Plata, Archuleta, San Juan and San Miguel counties, with partial funding from Great Outdoors Colorado (GOCO); and in 2004, public lands only in Dolores and Montezuma counties, funded entirely by the San Juan Public Lands. Funding for 2005 was again provided by San Juan Public Lands. The primary emphases for field work in 2005 were: 1. revisit and update information on rare plant occurrences of agency sensitive species in the Colorado Natural Heritage Program (CNHP) database that were last observed prior to 2000, in order to have the most current information available for informing the revision of the Resource Management Plan for the San Juan Public Lands (BLM and San Juan National Forest); 2.