Ultraviolet Light Detecting Beads UV-AST
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Understanding Infrared Light
TEACHER/PARENT ACTIVITY MANUAL Joint Polar Satellite System Understanding Infrared Light This activity educates students about the electromagnetic spectrum, or different forms of light detected by Earth observing satellites. The Joint Polar Satellite System (JPSS), a collaborative effort between NOAA and NASA, detects various wavelengths of the electromagnetic spectrum including infrared light to measure the temperature of Earth’s surface, oceans, and atmosphere. The data from these measurements provide the nation with accurate weather forecasts, hurricane warnings, wildfire locations, and much more! Provided is a list of materials that can be purchased to complete several learning activities, including simulating infrared light by constructing homemade infrared goggles. Learning Objectives Next Generation Science Standards (Grades 5–8) Performance Disciplinary Description Expectation Core Ideas 4-PS4-1 PS4.A: • Waves, which are regular patterns of motion, can be made in water Waves and Their Wave Properties by disturbing the surface. When waves move across the surface of Applications in deep water, the water goes up and down in place; there is no net Technologies for motion in the direction of the wave except when the water meets a Information Transfer beach. (Note: This grade band endpoint was moved from K–2.) • Waves of the same type can differ in amplitude (height of the wave) and wavelength (spacing between wave peaks). 4-PS4-2 PS4.B: An object can be seen when light reflected from its surface enters the Waves and Their Electromagnetic eyes. Applications in Radiation Technologies for Information Transfer 4-PS3-2 PS3.B: Light also transfers energy from place to place. -

Ultraviolet – Visible Spectroscopy for Determination of Α- and Β-Acids in Beer Hops Katharine Chau Lab Partner: Logan Bi
Ultraviolet – Visible Spectroscopy for Determination of α- and β-acids in Beer Hops Katharine Chau Lab Partner: Logan Billings TA: Kevin Fischer Date lab performed: 3/20/2018 Date report submitted: 4/9/2018 ABSTRACT: In this experiment, the content of α- and β-acids in beer hops is found through UV-Vis spectroscopic analysis. Three samples will be prepared by extracting finely grained hops through methanol and diluting with methanolic NaOH. The spectrums obtained give a constant overall shape. The experiment was done to find out the concentration of the third component from degraded α- and β-acids that is also existing in the hops samples with the help of the calculated concentrations of α- and β-acids. From the calculated results, the average concentration of the third component in all three samples was 0.061 g/L. INTRODUCTION: UV-Vis spectroscopy is a useful absorption or reflectance spectroscopy that helps determine the quantity of analytes by detecting the absorptivity or reflectance of a sample under ultra-violet to visible light wavelength range (1). In this experiment, the absorptivity of the samples were measured and the content of different components were determined from the spectrum. In this lab, UV-Vis spectroscopy was used in to obtain absorbance spectrums of α- and β- acids found in difference hops samples. The structures of α- and β-acids are shown as the Fig. 1 below. Figure 1. Structures of major α- and β-acids found in hops By understanding the content of α-acid in the hops, the bitterness flavor of beer can be controlled since the bitterness is formed by the iso-form of α-acid through isomization of α-acid. -

Relationship Between X-Ray and Ultraviolet Emission of Flares From
A&A 431, 679–686 (2005) Astronomy DOI: 10.1051/0004-6361:20041201 & c ESO 2005 Astrophysics Relationship between X-ray and ultraviolet emission of flares from dMe stars observed by XMM-Newton U. Mitra-Kraev1,L.K.Harra1,M.Güdel2, M. Audard3, G. Branduardi-Raymont1 ,H.R.M.Kay1,R.Mewe4, A. J. J. Raassen4,5, and L. van Driel-Gesztelyi1,6,7 1 Mullard Space Science Laboratory, University College London, Holmbury St. Mary, Dorking, Surrey RH5 6NT, UK e-mail: [email protected] 2 Paul Scherrer Institut, Würenlingen & Villigen, 5232 Villigen PSI, Switzerland 3 Columbia Astrophysics Laboratory, Columbia University, 550 West 120th Street, New York, NY 10027, USA 4 SRON National Institute for Space Research, Sorbonnelaan 2, 3584 CA Utrecht, The Netherlands 5 Astronomical Institute “Anton Pannekoek”, Kruislaan 403, 1098 SJ Amsterdam, The Netherlands 6 Observatoire de Paris, LESIA, 92195 Meudon, France 7 Konkoly Observatory, 1525 Budapest, Hungary Received 30 April 2004 / Accepted 30 September 2004 Abstract. We present simultaneous ultraviolet and X-ray observations of the dMe-type flaring stars AT Mic, AU Mic, EV Lac, UV Cet and YZ CMi obtained with the XMM-Newton observatory. During 40 h of simultaneous observation we identify 13 flares which occurred in both wave bands. For the first time, a correlation between X-ray and ultraviolet flux for stellar flares has been observed. We find power-law relationships between these two wavelength bands for the flare luminosity increase, as well as for flare energies, with power-law exponents between 1 and 2. We also observe a correlation between the ultraviolet flare energy and the X-ray luminosity increase, which is in agreement with the Neupert effect and demonstrates that chromospheric evaporation is taking place. -

Ultraviolet and Green Light Cause Different Types of Damage in Rat Retina
Ultraviolet and Green Light Cause Different Types of Damage in Rat Retina Theo G. M. F. Gorgels? and Dirk van Norren*^ Purpose. To assess the influence of wavelength on retinal light damage in rat with funduscopy and histology and to determine a detailed action spectrum. Methods. Adult Long Evans rats were anesthetized, and small patches of retina were exposed to narrow-band irradiations in the range of 320 to 600 nm using a Xenon arc and Maxwellian view conditions. After 3 days, the retina was examined with funduscopy and prepared for light microscopy. Results. The dose that produced a change just visible in fundo was determined for each wavelength. This threshold dose for funduscopic damage increased monotonically from 0.35 J/cm2 at 320 nm to 1600 J/cm2 at 550 nm. At 600 nm, exposure of more than 3000J/cnr did not cause funduscopic damage. Morphologic changes in retinas exposed to threshold doses at wavelengths from 320 to 440 nm were similar and consisted of pyknosis of photorecep- tors. Retinas exposed to threshold doses of 470 to 550 nm had different morphologic appear- ances. Retinal pigment epithelial cells were swollen, and their melanin had lost the characteris- tic apical distribution. Some pyknosis was found in photoreceptors. Conclusions. Damage sensitivity in rat increases enormously from visible to ultraviolet wave- lengths. Compelling evidence is presented that two morphologically distinct types of damage occur in the rat retina, depending on the wavelength. Because two types also have been described in monkey, a remarkable similarity seems to exist across species. Invest Ophthahnol VisSci. -

Xcited from Its Ground State to an Electronic Excited State
Chapter 1: UV-Visible & Fluorescence Spectroscopy This chapter covers two methods of spectroscopic characterization: UV-Visible absorption spectroscopy (often abbreviated as UV-Vis) and fluorescence emission spectroscopy. These two methods are measured over the same range of wavelengths, but are caused by two different phenomena. In both cases, the wavelengths used are the near-ultraviolet range (200 to 400 nm) and the visible range (400 to 750 nm). These two regions are typically considered together as a single category because there is a common physical basis for the behavior of a compound in both UV and visible light. UV-Vis measures the absorption of light in this range, while fluorescence measures the light emitted by a sample in this range after absorbing light at a higher energy than it is emitting. 1.1 UV-Visible Spectroscopy UV-Visible absorption spectroscopy involves measuring the absorbance of light by a compound as a function of wavelength in the UV-visible range. When a molecule absorbs a photon of UV-Vis light, the molecule is excited from its ground state to an electronic excited state. In other words, an electron is promoted from the HOMO (Highest-energy Occupied Molecular Orbital) of the molecule to the LUMO (Lowest-energy Unoccupied Molecular Orbital) of the molecule. The HOMO is commonly a π orbital of a conjugated functional group and the LUMO is commonly a π* orbital of the conjugated functional group. The smaller the energy difference between HOMO and LUMO, the less energy is needed and the longer the wavelength that will be absorbed (Figure 1-1). -

Learn More About X-Rays, CT Scans and Mris (Pdf)
What is the difference between X-Rays, CT Scans, and MRIs? X-Rays are a form of electromagnetic radiation, like light. They are less energetic than gamma rays, and more energetic than ultraviolet light. Because they pass easily through soft tissue, like organs and muscles, but not so easily through hard tissue like bones and teeth, we are most familiar with them being used to look at skeletal structures. Sometimes a person ingests or has injected an X-ray opaque fluid that will fill a space of interest for X-ray imaging. This is called an angiogram. A nuclear scan uses an injected gamma ray emitting substance that accumulates in the organ of interest and a special camera records the gamma rays. A CT Scan is usually a series of X-rays taken from different directions that are then assembled into a three dimensional model of the subject in a computer. CT stands for computed tomography, and tomography means a picture of a slice. Where an X-ray may show edges of soft tissues all stacked on top of each other, the computer in a CT scan can figure out how those edges relate to each other in depth, and so the image has much more soft tissue usability. Another kind of CT scan uses positrons. I have to mention this because positrons are antimatter electrons (Yes, antimatter does exist and it is useful!) In Positon Emission Tomography (PET) a positron emitting radionuclide (radioactive material) is attached to a metabolically useful molecule. This is introduced to the tissue, and as emitted positrons decompose they emit gamma rays which can be traced by the machine and computer back to their points of origin, and an image is formed. -

24 Electromagnetic Waves.Pdf
CHAPTER 24 | ELECTROMAGNETIC WAVES 861 24 ELECTROMAGNETIC WAVES Figure 24.1 Human eyes detect these orange “sea goldie” fish swimming over a coral reef in the blue waters of the Gulf of Eilat (Red Sea) using visible light. (credit: Daviddarom, Wikimedia Commons) Learning Objectives 24.1. Maxwell’s Equations: Electromagnetic Waves Predicted and Observed • Restate Maxwell’s equations. 24.2. Production of Electromagnetic Waves • Describe the electric and magnetic waves as they move out from a source, such as an AC generator. • Explain the mathematical relationship between the magnetic field strength and the electrical field strength. • Calculate the maximum strength of the magnetic field in an electromagnetic wave, given the maximum electric field strength. 24.3. The Electromagnetic Spectrum • List three “rules of thumb” that apply to the different frequencies along the electromagnetic spectrum. • Explain why the higher the frequency, the shorter the wavelength of an electromagnetic wave. • Draw a simplified electromagnetic spectrum, indicating the relative positions, frequencies, and spacing of the different types of radiation bands. • List and explain the different methods by which electromagnetic waves are produced across the spectrum. 24.4. Energy in Electromagnetic Waves • Explain how the energy and amplitude of an electromagnetic wave are related. • Given its power output and the heating area, calculate the intensity of a microwave oven’s electromagnetic field, as well as its peak electric and magnetic field strengths Introduction to Electromagnetic Waves The beauty of a coral reef, the warm radiance of sunshine, the sting of sunburn, the X-ray revealing a broken bone, even microwave popcorn—all are brought to us by electromagnetic waves. -

Development of Plasmonically Cloaked Nanoparticles
Project No. 11-3248 Development of Plasmonically Cloaked Nanoparticles Fuel Cycle Dr. Eric Burge, Idaho State University In collaboraon with: Georgia Instute of Technology University of Maryland Daniel Vega, Federal POC Keith Jewell, Technical POC Development of Plasmonically Cloaked Nanoparticles Final Report [Intentional Blank Page] Development of Plasmonically Cloaked Nanoparticles 2 Approved By: ___________________________________________________ Dr. Eric Burgett, Idaho State University ___________________________________________________ Dr. Christopher Summers, Georgia Institute of Technology ___________________________________________________ Dr. Mohamad Al-Sheikhly, University of Maryland Development of Plasmonically Cloaked Nanoparticles 3 [Intentional Blank Page] Development of Plasmonically Cloaked Nanoparticles 4 Table of Contents TABLE OF FIGURES 7 INTRODUCTION 9 PROPOSED SCOPE DESCRIPTION 9 PLASMONIC CLOAK THEORY 9 NEUTRON DETECTION USING METAMATERIALS 10 FLUIDIZED BED REACTOR DESIGN 13 DESIGN OF LARGE AREA ARRAY 14 CURRENT STATUS : 14 DELIVERABLE #1 DOCUMENT REPORTING THE RESPONSE OF THE FIRST PLANAR DEVICES AND INITIAL GROWTH QUALITY OF NEW DETECTOR SYSTEM PROVIDED TO DOE. 14 DELIVERABLE #2 DOCUMENT REPORTING THE RESPONSE OF THE FIRST BAND GAP ENGINEERED SAMPLE DEVICES AND INITIAL GROWTH QUALITY OF NEW DETECTOR SYSTEM PROVIDED TO DOE. 14 DELIVERABLE #3 DOCUMENT REPORTING FIRST NEUTRON SENSOR PERFORMANCE. 19 DELIVERABLE #4 DOCUMENT REPORTING THE OPTIMIZED RESPONSE OF THE FIRST PLANAR NEUTRON DEVICES AND INITIAL GROWTH QUALITY OF NEW DETECTOR SYSTEM PROVIDED TO DOE 19 DELIVERABLE #5 DOCUMENT REPORTING THE COMMISSIONING OF THE REACTOR DATA FOR THE FIRST CLOAKED DEVICES 19 DELIVERABLE #6 BEGIN TO DEVELOP FIRST CLOAKED DEVICES 19 DELIVERABLE #7 DOCUMENT REPORTING THE FIRST CLOAKED NANOPARTICLE BASED RADIATION TESTS DEVELOPED 28 DELIVERABLE #8 DOCUMENT REPORTING THE DESIGN AND POTENTIAL PERFORMANCE OF PROTON RECOIL VERSIONS OF CLOAKED NANOPARTICLES DEVELOPED. -

REVERSIBLE COLOR MODIFICATION of BLUE ZIRCON by LONG-WAVE ULTRAVIOLET RADIATION Nathan D
FEATURE AR ICLES REVERSIBLE COLOR MODIFICATION OF BLUE ZIRCON BY LONG-WAVE ULTRAVIOLET RADIATION Nathan D. Renfro Exposing blue zircon to long-wave ultraviolet (LWUV) radiation introduces a brown coloration, resulting in a somewhat unattractive, much less valuable gemstone. Common sources of accidental long-wave radiation that can affect mounted faceted blue zircons are tanning beds and UV lights used to apply acrylic fingernails. To determine if the LWUV-induced brown color in zircon is completely and easily reversible, quantitative UV-visible spectroscopy was used to measure the difference in absorption before and after LWUV exposure. This study explored the nature of the UV-induced color-causing defect to es- tablish whether subsequent exposure to visible light would completely restore the blue color. Spectro- scopic examination showed that blue color in zircon is due to a broad absorption band in the ordinary ray, starting at 500 nm and centered at approximately 650 nm. LWUV exposure induced absorption features, including a broad band centered at 485 nm that was responsible for the brown color. irconium silicate (ZrSiO ), better known as zir- cern was returning the stones to their vivid blue color. 4 con, is prized for its brilliance, vibrant color, One of these stones was reportedly restored to the de- Zand high clarity. Though commonly thought sired blue, while the status of the other is unknown. of as a colorless diamond simulant, this tetragonal In the case of a blue zircon that turned brown mineral occurs in several countries in a wide variety (Koivula and Misiorowski, 1986), the blue color was of colors. -

UV Radiation (PDF)
United States Air and Radiation EPA 430-F-10-025 Environmental Protection 6205J June 2010 Agency www.epa.gov/ozone/strathome.html UV Radiation This fact sheet explains the types of ultraviolet radiation and the various factors that can affect the levels reaching the Earth’s surface. The sun emits energy over a broad spectrum of wavelengths: visible light that you see, infrared radiation that you feel as heat, and ultraviolet (UV) radiation that you can’t see or feel. UV radiation has a shorter wavelength and higher energy than visible light. It affects human health both positively and negatively. Short exposure to UVB radiation generates vitamin D, but can also lead to sunburn depending on an individual’s skin type. Fortunately for life on Earth, our atmosphere’s stratospheric ozone layer shields us from most UV radiation. What does get through the ozone layer, however, can cause the following problems, particularly for people who spend unprotected time outdoors: ● Skin cancer ● Suppression of the immune system ● Cataracts ● Premature aging of the skin Did You Since the benefits of sunlight cannot be separated from its damaging effects, it is important to understand the risks of overexposure, and take simple precautions to protect yourself. Know? Types of UV Radiation Ultraviolet (UV) radiation, from the Scientists classify UV radiation into three types or bands—UVA, UVB, and UVC. The ozone sun and from layer absorbs some, but not all, of these types of UV radiation: ● tanning beds, is UVA: Wavelength: 320-400 nm. Not absorbed by the ozone layer. classified as a ● UVB: Wavelength: 290-320 nm. -

The Greenhouse Effect: Impacts of Ultraviolet-B (UV-B) Radiation, Carbon Dioxide (CO2), and Ozone (O3) on Vegetation
Enrironmental Pollution 61 (1989) 263-393 The Greenhouse Effect: Impacts of Ultraviolet-B (UV-B) Radiation, Carbon Dioxide (CO2), and Ozone (O3) on Vegetation S. V. Krupa Department of Plant Pathology, University of Minnesota, St Paul, MN 55108, USA & R. N. Kickert 4151 NW Jasmine Place, Corvallis, OR 97330, USA (Received 8 May 1989; accepted 19 June 1989) ABSTRACT There is a fast growing and an extremely serious international scientific, public and political concern regarding man's influence on the global climate. The decrease in stratospheric ozone (03) and the consequent possible increase in ultraviolet-B ( UV-B) is a critical issue. In addition, tropospheric concentrations of 'greenhouse gases' such as carbon dioxide (C02), nitrous oxide ( N 2O) and methane (CH4) are increasing. These phenomena, coupled with man's use of chlorofluorocarbons ( CFCs), chlorocarbons ( CCs), and organo-bromines ( OBs) are considered to result in the modification of the earth's 0 3 column and altered interactions between the stratosphere and the troposphere. A result of such interactions couM be the global warming. As opposed to these processes, tropospheric 03 concentrations appear to be increasing in some parts of the world (e.g. North America). Such tropospheric increases in 0 3 and particulate matter may offset any predicted increases in UV-B at those locations. Presently most general circulation models ( GCMs ) used to predict climate change are one- or two-dimensional models. Application of satisfactory three- dimensional models is limited by the available computer power. Recent studies 263 Environ. Poilut. 0269-7491/89/$03-50 © 1989 Elsevier Science Publishers Ltd, England. -
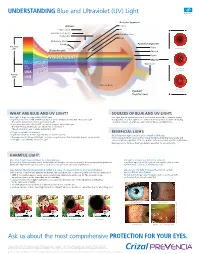
UNDERSTANDING Blue and Ultraviolet (UV) Light Ask Us About
UNDERSTANDING Blue and Ultraviolet (UV) Light Anterior Segment: Adnexa: Cornea Upper eyelid Iris Obicularis oculi muscle Crystalline lens Conjunctiva Pupil Meibomian gland Eyelash Posterior Segment: Beneficial Sclera Light Normal Macula Wavelength Choroid VISIBLE LIGHT Retina Macula BLUE LE1 SLEEP CYC TURQUOISE Optic nerve 2 BLUE AMD VIOLET Dry Macular 3 CT Degeneration ARA UVA CAT Harmful Light UVB UVC Vitreous body Wet Macular Eyeball Degeneration (Sagittal view) WHAT ARE BLUE AND UV LIGHT? SOURCES OF BLUE AND UV LIGHT: Blue light is high-energy visible (HEV) light. Blue light and UV light are present all year and in any weather condition (sunny, • Light waves between 465 and 495 nanometers (nm) contain beneficial, Blue-Turquoise light. cloudy, rainy, etc.). Blue light is also emitted from many modern devices including This light is beneficial for vision and overall health. computers, tablets, smartphones and compact fluorescent light bulbs. • Light waves between 415 and 455 nm contain harmful, Blue-Violet light. This High-Energy Visible light can induce retinal cell death.* *Based on in-vitro tests of swine (pig) retinal cells4 UV light is invisible to humans. BENEFICIAL LIGHT: • Light waves that are shorter than 380 nm are classified as UV. Blue-Turquoise light is essential for overall well-being. • Overexposure to UVA and UVB light can have a negative and often irreversible impact on eye health. It is necessary for both visual and non-visual functions, including visual acuity and • UVC light is absorbed by the Ozone Layer. color perception, regulation of the sleep/wake cycle, mood, and cognitive performance. Exposing eyes to this beneficial light daily is important for overall health.