A Tool for Interrogation of Macromolecular Structure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Density Functional Theory
Density Functional Approach Francesco Sottile Ecole Polytechnique, Palaiseau - France European Theoretical Spectroscopy Facility (ETSF) 22 October 2010 Density Functional Theory 1. Any observable of a quantum system can be obtained from the density of the system alone. < O >= O[n] Hohenberg, P. and W. Kohn, 1964, Phys. Rev. 136, B864 Density Functional Theory 1. Any observable of a quantum system can be obtained from the density of the system alone. < O >= O[n] 2. The density of an interacting-particles system can be calculated as the density of an auxiliary system of non-interacting particles. Hohenberg, P. and W. Kohn, 1964, Phys. Rev. 136, B864 Kohn, W. and L. Sham, 1965, Phys. Rev. 140, A1133 Density Functional ... Why ? Basic ideas of DFT Importance of the density Example: atom of Nitrogen (7 electron) 1. Any observable of a quantum Ψ(r1; ::; r7) 21 coordinates system can be obtained from 10 entries/coordinate ) 1021 entries the density of the system alone. 8 bytes/entry ) 8 · 1021 bytes 4:7 × 109 bytes/DVD ) 2 × 1012 DVDs 2. The density of an interacting-particles system can be calculated as the density of an auxiliary system of non-interacting particles. Density Functional ... Why ? Density Functional ... Why ? Density Functional ... Why ? Basic ideas of DFT Importance of the density Example: atom of Oxygen (8 electron) 1. Any (ground-state) observable Ψ(r1; ::; r8) 24 coordinates of a quantum system can be 24 obtained from the density of the 10 entries/coordinate ) 10 entries 8 bytes/entry ) 8 · 1024 bytes system alone. 5 · 109 bytes/DVD ) 1015 DVDs 2. -

Open Babel Documentation Release 2.3.1
Open Babel Documentation Release 2.3.1 Geoffrey R Hutchison Chris Morley Craig James Chris Swain Hans De Winter Tim Vandermeersch Noel M O’Boyle (Ed.) December 05, 2011 Contents 1 Introduction 3 1.1 Goals of the Open Babel project ..................................... 3 1.2 Frequently Asked Questions ....................................... 4 1.3 Thanks .................................................. 7 2 Install Open Babel 9 2.1 Install a binary package ......................................... 9 2.2 Compiling Open Babel .......................................... 9 3 obabel and babel - Convert, Filter and Manipulate Chemical Data 17 3.1 Synopsis ................................................. 17 3.2 Options .................................................. 17 3.3 Examples ................................................. 19 3.4 Differences between babel and obabel .................................. 21 3.5 Format Options .............................................. 22 3.6 Append property values to the title .................................... 22 3.7 Filtering molecules from a multimolecule file .............................. 22 3.8 Substructure and similarity searching .................................. 25 3.9 Sorting molecules ............................................ 25 3.10 Remove duplicate molecules ....................................... 25 3.11 Aliases for chemical groups ....................................... 26 4 The Open Babel GUI 29 4.1 Basic operation .............................................. 29 4.2 Options ................................................. -

Open Data, Open Source, and Open Standards in Chemistry: the Blue Obelisk Five Years On" Journal of Cheminformatics Vol
Oral Roberts University Digital Showcase College of Science and Engineering Faculty College of Science and Engineering Research and Scholarship 10-14-2011 Open Data, Open Source, and Open Standards in Chemistry: The lueB Obelisk five years on Andrew Lang Noel M. O'Boyle Rajarshi Guha National Institutes of Health Egon Willighagen Maastricht University Samuel Adams See next page for additional authors Follow this and additional works at: http://digitalshowcase.oru.edu/cose_pub Part of the Chemistry Commons Recommended Citation Andrew Lang, Noel M O'Boyle, Rajarshi Guha, Egon Willighagen, et al.. "Open Data, Open Source, and Open Standards in Chemistry: The Blue Obelisk five years on" Journal of Cheminformatics Vol. 3 Iss. 37 (2011) Available at: http://works.bepress.com/andrew-sid-lang/ 19/ This Article is brought to you for free and open access by the College of Science and Engineering at Digital Showcase. It has been accepted for inclusion in College of Science and Engineering Faculty Research and Scholarship by an authorized administrator of Digital Showcase. For more information, please contact [email protected]. Authors Andrew Lang, Noel M. O'Boyle, Rajarshi Guha, Egon Willighagen, Samuel Adams, Jonathan Alvarsson, Jean- Claude Bradley, Igor Filippov, Robert M. Hanson, Marcus D. Hanwell, Geoffrey R. Hutchison, Craig A. James, Nina Jeliazkova, Karol M. Langner, David C. Lonie, Daniel M. Lowe, Jerome Pansanel, Dmitry Pavlov, Ola Spjuth, Christoph Steinbeck, Adam L. Tenderholt, Kevin J. Theisen, and Peter Murray-Rust This article is available at Digital Showcase: http://digitalshowcase.oru.edu/cose_pub/34 Oral Roberts University From the SelectedWorks of Andrew Lang October 14, 2011 Open Data, Open Source, and Open Standards in Chemistry: The Blue Obelisk five years on Andrew Lang Noel M O'Boyle Rajarshi Guha, National Institutes of Health Egon Willighagen, Maastricht University Samuel Adams, et al. -

A Nano-Visualization Software for Education and Research
A Nano-Visualization Software for Education and Research Lillian C. Oetting Department of Computer Science, Stanford University, Stanford, CA 94305 USA West High School, Iowa City, IA 52246 USA Tehseen Z. Raza Department of Physics and Astronomy, University of Iowa, Iowa City, IA 52242 USA Hassan Raza Department of Electrical and Computer Engineering, University of Iowa, Iowa City, IA 52242 USA Centre for Fundamental Research, Islamabad, Pakistan Abstract: We report the development of a user-friendly nano-visualization software program which can acquaint high-school students with nanotechnology. The visual introduction to atoms and molecules, which are the building blocks of this technology, is an effective way to introduce the key concepts in this area. The software’s graphical user interface enables multidimensional atomic visualization by using ball and stick schematics. Additionally, the software provides the option of wavefunction visualization for arbitrary nanomaterials and nanostructures by using extended Hückel theory. The software is instructive, application oriented and may be useful not only in high school education but also for the undergraduate research and teaching. 1 I. Introduction: The ability to accurately depict atomic, molecular and electronic structures has been a key factor in the advancement of nanotechnology. In this context, it is imperative to provide teaching and research platforms to motivate students towards this novel technology [1-3], while keeping the societal implications in perspective [4]. Nano-visualization appeals well due to its simplistic, yet elegant approach towards the visual representation of detailed concepts about quantum mechanics, quantum chemistry and linear algebra. Additionally, the conflux of quantum mechanics, numerical computation, graphical design, and computer programming gives exposure to the multi-disciplinary aspect of this technology [5-8]. -

Computer-Assisted Catalyst Development Via Automated Modelling of Conformationally Complex Molecules
www.nature.com/scientificreports OPEN Computer‑assisted catalyst development via automated modelling of conformationally complex molecules: application to diphosphinoamine ligands Sibo Lin1*, Jenna C. Fromer2, Yagnaseni Ghosh1, Brian Hanna1, Mohamed Elanany3 & Wei Xu4 Simulation of conformationally complicated molecules requires multiple levels of theory to obtain accurate thermodynamics, requiring signifcant researcher time to implement. We automate this workfow using all open‑source code (XTBDFT) and apply it toward a practical challenge: diphosphinoamine (PNP) ligands used for ethylene tetramerization catalysis may isomerize (with deleterious efects) to iminobisphosphines (PPNs), and a computational method to evaluate PNP ligand candidates would save signifcant experimental efort. We use XTBDFT to calculate the thermodynamic stability of a wide range of conformationally complex PNP ligands against isomeriation to PPN (ΔGPPN), and establish a strong correlation between ΔGPPN and catalyst performance. Finally, we apply our method to screen novel PNP candidates, saving signifcant time by ruling out candidates with non‑trivial synthetic routes and poor expected catalytic performance. Quantum mechanical methods with high energy accuracy, such as density functional theory (DFT), can opti- mize molecular input structures to a nearby local minimum, but calculating accurate reaction thermodynamics requires fnding global minimum energy structures1,2. For simple molecules, expert intuition can identify a few minima to focus study on, but an alternative approach must be considered for more complex molecules or to eventually fulfl the dream of autonomous catalyst design 3,4: the potential energy surface must be frst surveyed with a computationally efcient method; then minima from this survey must be refned using slower, more accurate methods; fnally, for molecules possessing low-frequency vibrational modes, those modes need to be treated appropriately to obtain accurate thermodynamic energies 5–7. -

Camcasp 5.9 Alston J. Misquitta† and Anthony J
CamCASP 5.9 Alston J. Misquittay and Anthony J. Stoneyy yDepartment of Physics and Astronomy, Queen Mary, University of London, 327 Mile End Road, London E1 4NS yy University Chemical Laboratory, Lensfield Road, Cambridge CB2 1EW September 1, 2015 Abstract CamCASP is a suite of programs designed to calculate molecular properties (multipoles and frequency- dependent polarizabilities) in single-site and distributed form, and interaction energies between pairs of molecules, and thence to construct atom–atom potentials. The CamCASP distribution also includes the programs Pfit, Casimir,Gdma 2.2, Cluster, and Process. Copyright c 2007–2014 Alston J. Misquitta and Anthony J. Stone Contents 1 Introduction 1 1.1 Authors . .1 1.2 Citations . .1 2 What’s new? 2 3 Outline of the capabilities of CamCASP and other programs 5 3.1 CamCASP limits . .7 4 Installation 7 4.1 Building CamCASP from source . .9 5 Using CamCASP 10 5.1 Workflows . 10 5.2 High-level scripts . 10 5.3 The runcamcasp.py script . 11 5.4 Low-level scripts . 13 6 Data conventions 13 7 CLUSTER: Detailed specification 14 7.1 Prologue . 15 7.2 Molecule definitions . 15 7.3 Geometry manipulations and other transformations . 16 7.4 Job specification . 18 7.5 Energy . 23 7.6 Crystal . 25 7.7 ORIENT ............................................... 26 7.8 Finally, . 29 8 Examples 29 8.1 A SAPT(DFT) calculation . 29 8.2 An example properties calculation . 30 8.3 Dispersion coefficients . 34 8.4 Using CLUSTER to obtain the dimer geometry . 36 9 CamCASP program specification 39 9.1 Global data . -

Designing Universal Chemical Markup (UCM) Through the Reusable Methodology Based on Analyzing Existing Related Formats
Designing Universal Chemical Markup (UCM) through the reusable methodology based on analyzing existing related formats Background: In order to design concepts for a new general-purpose chemical format we analyzed the strengths and weaknesses of current formats for common chemical data. While the new format is discussed more in the next article, here we describe our software s t tools and two stage analysis procedure that supplied the necessary information for the n i r development. The chemical formats analyzed in both stages were: CDX, CDXML, CML, P CTfile and XDfile. In addition the following formats were included in the first stage only: e r P CIF, InChI, NCBI ASN.1, NCBI XML, PDB, PDBx/mmCIF, PDBML, SMILES, SLN and Mol2. Results: A two stage analysis process devised for both XML (Extensible Markup Language) and non-XML formats enabled us to verify if and how potential advantages of XML are utilized in the widely used general-purpose chemical formats. In the first stage we accumulated information about analyzed formats and selected the formats with the most general-purpose chemical functionality for the second stage. During the second stage our set of software quality requirements was used to assess the benefits and issues of selected formats. Additionally, the detailed analysis of XML formats structure in the second stage helped us to identify concepts in those formats. Using these concepts we came up with the concise structure for a new chemical format, which is designed to provide precise built-in validation capabilities and aims to avoid the potential issues of analyzed formats. -

Python Tools in Computational Chemistry (And Biology)
Python Tools in Computational Chemistry (and Biology) Andrew Dalke Dalke Scientific, AB Göteborg, Sweden EuroSciPy, 26-27 July, 2008 “Why does ‘import numpy’ take 0.4 seconds? Does it need to import 228 libraries?” - My first Numpy-discussion post (paraphrased) Your use case isn't so typical and so suffers on the import time end of the balance. - Response from Robert Kern (Others did complain. Import time down to 0.28s.) 52,000 structures PDB doubles every 2½ years HEADER PHOTORECEPTOR 23-MAY-90 1BRD 1BRD 2 COMPND BACTERIORHODOPSIN 1BRD 3 SOURCE (HALOBACTERIUM $HALOBIUM) 1BRD 4 EXPDTA ELECTRON DIFFRACTION 1BRD 5 AUTHOR R.HENDERSON,J.M.BALDWIN,T.A.CESKA,F.ZEMLIN,E.BECKMANN, 1BRD 6 AUTHOR 2 K.H.DOWNING 1BRD 7 REVDAT 3 15-JAN-93 1BRDB 1 SEQRES 1BRDB 1 REVDAT 2 15-JUL-91 1BRDA 1 REMARK 1BRDA 1 .. ATOM 54 N PRO 8 20.397 -15.569 -13.739 1.00 20.00 1BRD 136 ATOM 55 CA PRO 8 21.592 -15.444 -12.900 1.00 20.00 1BRD 137 ATOM 56 C PRO 8 21.359 -15.206 -11.424 1.00 20.00 1BRD 138 ATOM 57 O PRO 8 21.904 -15.930 -10.563 1.00 20.00 1BRD 139 ATOM 58 CB PRO 8 22.367 -14.319 -13.591 1.00 20.00 1BRD 140 ATOM 59 CG PRO 8 22.089 -14.564 -15.053 1.00 20.00 1BRD 141 ATOM 60 CD PRO 8 20.647 -15.054 -15.103 1.00 20.00 1BRD 142 ATOM 61 N GLU 9 20.562 -14.211 -11.095 1.00 20.00 1BRD 143 ATOM 62 CA GLU 9 20.192 -13.808 -9.737 1.00 20.00 1BRD 144 ATOM 63 C GLU 9 19.567 -14.935 -8.932 1.00 20.00 1BRD 145 ATOM 64 O GLU 9 19.815 -15.104 -7.724 1.00 20.00 1BRD 146 ATOM 65 CB GLU 9 19.248 -12.591 -9.820 1.00 99.00 1 1BRD 147 ATOM 66 CG GLU 9 19.902 -11.351 -10.387 1.00 99.00 1 1BRD 148 ATOM 67 CD GLU 9 19.243 -10.169 -10.980 1.00 99.00 1 1BRD 149 ATOM 68 OE1 GLU 9 18.323 -10.191 -11.782 1.00 99.00 1 1BRD 150 ATOM 69 OE2 GLU 9 19.760 -9.089 -10.597 1.00 99.00 1 1BRD 151 ATOM 70 N TRP 10 18.764 -15.737 -9.597 1.00 20.00 1BRD 152 ATOM 71 CA TRP 10 18.034 -16.884 -9.090 1.00 20.00 1BRD 153 ATOM 72 C TRP 10 18.843 -17.908 -8.318 1.00 20.00 1BRD 154 ATOM 73 O TRP 10 18.376 -18.310 -7.230 1.00 20.00 1BRD 155 . -

Open Data, Open Source and Open Standards in Chemistry: the Blue Obelisk five Years On
Open Data, Open Source and Open Standards in chemistry: The Blue Obelisk ¯ve years on Noel M O'Boyle¤1 , Rajarshi Guha2 , Egon L Willighagen3 , Samuel E Adams4 , Jonathan Alvarsson5 , Richard L Apodaca6 , Jean-Claude Bradley7 , Igor V Filippov8 , Robert M Hanson9 , Marcus D Hanwell10 , Geo®rey R Hutchison11 , Craig A James12 , Nina Jeliazkova13 , Andrew SID Lang14 , Karol M Langner15 , David C Lonie16 , Daniel M Lowe4 , J¶er^omePansanel17 , Dmitry Pavlov18 , Ola Spjuth5 , Christoph Steinbeck19 , Adam L Tenderholt20 , Kevin J Theisen21 , Peter Murray-Rust4 1Analytical and Biological Chemistry Research Facility, Cavanagh Pharmacy Building, University College Cork, College Road, Cork, Co. Cork, Ireland 2NIH Center for Translational Therapeutics, 9800 Medical Center Drive, Rockville, MD 20878, USA 3Division of Molecular Toxicology, Institute of Environmental Medicine, Nobels vaeg 13, Karolinska Institutet, 171 77 Stockholm, Sweden 4Unilever Centre for Molecular Sciences Informatics, Department of Chemistry, University of Cambridge, Lens¯eld Road, CB2 1EW, UK 5Department of Pharmaceutical Biosciences, Uppsala University, Box 591, 751 24 Uppsala, Sweden 6Metamolecular, LLC, 8070 La Jolla Shores Drive #464, La Jolla, CA 92037, USA 7Department of Chemistry, Drexel University, 32nd and Chestnut streets, Philadelphia, PA 19104, USA 8Chemical Biology Laboratory, Basic Research Program, SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD 21702, USA 9St. Olaf College, 1520 St. Olaf Ave., North¯eld, MN 55057, USA 10Kitware, Inc., 28 Corporate Drive, Clifton Park, NY 12065, USA 11Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA 12eMolecules Inc., 380 Stevens Ave., Solana Beach, California 92075, USA 13Ideaconsult Ltd., 4.A.Kanchev str., So¯a 1000, Bulgaria 14Department of Engineering, Computer Science, Physics, and Mathematics, Oral Roberts University, 7777 S. -

Chemdoodle Web Components: HTML5 Toolkit for Chemical Graphics, Interfaces, and Informatics Melanie C Burger1,2*
Burger. J Cheminform (2015) 7:35 DOI 10.1186/s13321-015-0085-3 REVIEW Open Access ChemDoodle Web Components: HTML5 toolkit for chemical graphics, interfaces, and informatics Melanie C Burger1,2* Abstract ChemDoodle Web Components (abbreviated CWC, iChemLabs, LLC) is a light-weight (~340 KB) JavaScript/HTML5 toolkit for chemical graphics, structure editing, interfaces, and informatics based on the proprietary ChemDoodle desktop software. The library uses <canvas> and WebGL technologies and other HTML5 features to provide solutions for creating chemistry-related applications for the web on desktop and mobile platforms. CWC can serve a broad range of scientific disciplines including crystallography, materials science, organic and inorganic chemistry, biochem- istry and chemical biology. CWC is freely available for in-house use and is open source (GPL v3) for all other uses. Keywords: ChemDoodle Web Components, Chemical graphics, Animations, Cheminformatics, HTML5, Canvas, JavaScript, WebGL, Structure editor, Structure query Introduction Mobile browsers did support HTML5, which opened How we communicate chemical information is increas- the door to web applications built with only HTML, ingly technology driven. Learning management systems, CSS and JavaScript (JS), such as the ChemDoodle Web virtual classrooms and MOOCs are a few examples where Components. chemistry educators need forward compatible tools for digital natives. Companies that implement emerg- Review ing web technologies can find efficiencies and benefit The ChemDoodle Web Components technology stack from competitive advantages. The first chemical graph- and features ics toolkit for the web, MDL Chime, was introduced in The ChemDoodle Web Components library, released in 1996 [1]. Based on the molecular visualization program 2009, is the first chemistry toolkit for structure viewing RasMol, Chime was developed as a plugin for Netscape and editing that is originally built using only web stand- and later for Internet Explorer and Firefox. -
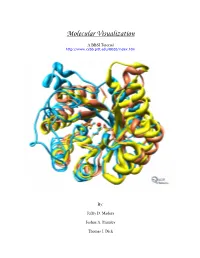
Instructions for PDB Downloading (From Either Website)
Molecular Visualization A BBSI Tutorial http://www.ccbb.pitt.edu/BBSI/index.htm By: Jeffry D. Madura Joshua A. Plumley Thomas J. Dick Table of Contents Instructions for PDB downloading..........................................................2 RasMol Tutorial ...........................................................................................3 VMD Tutorial ...............................................................................................5 CAChe Tutuorial..........................................................................................8 MOE Tutorial ............................................................................................. 10 Chimera Tutorial ....................................................................................... 13 Exercises .................................................................................................... 16 1 Instructions for PDB downloading (from either website) -Go to: - Go to: www.rcsb.org/pdb/ www.pdb.bu.edu/oca-bin/pdblite -Type in name of protein (examples at - Type in name of Protein / bottom of the page). Macromolecule ( examples at bottom of page). - Click on Name icon (first name in purple box). - Click on Retrieve Released Data Matching Your Query icon. -On the left side of the screen, click on Download/Display Structure - Highlight any of the molecule name and click on the View/ Analyze/ -Under Download the Structure File, Save Macro Molecule icon. right click on the X where the PDB(top) meets with none, under - Under the Data Retrieval section, -

USER MANUAL Version 4.5
GROMACS Groningen Machine for Chemical Simulations USER MANUAL Version 4.5 GROMACS USER MANUAL Version 4.5 Written by Emile Apol, Rossen Apostolov, Herman J.C. Berendsen, Aldert van Buuren, Par¨ Bjelkmar, Rudi van Drunen, Anton Feenstra, Gerrit Groenhof, Peter Kasson, Per Larsson, Peiter Meulenhoff, Teemu Murtola, Szilard´ Pall,´ Sander Pronk, Roland Schultz, Michael Shirts, Alfons Sijbers, Peter Tieleman Berk Hess, David van der Spoel, and Erik Lindahl. Additional contributions by Mark Abraham, Christoph Junghans, Carsten Kutzner, Justin A. Lemkul, Erik Marklund, Maarten Wolf. c 1991–2000: Department of Biophysical Chemistry, University of Groningen. Nijenborgh 4, 9747 AG Groningen, The Netherlands. c 2001–2010: The GROMACS development teams at the Royal Institute of Technology and Uppsala University, Sweden. More information can be found on our website: www.gromacs.org. iv Preface & Disclaimer This manual is not complete and has no pretention to be so due to lack of time of the contributors – our first priority is to improve the software. It is worked on continuously, which in some cases might mean the information is not entirely correct. Comments are welcome, please send them by e-mail to [email protected], or to one of the mailing lists (see www.gromacs.org). We try to release an updated version of the manual whenever we release a new version of the soft- ware, so in general it is a good idea to use a manual with the same major and minor release number as your GROMACS installation. Any revision numbers (like 3.1.1) are however independent, to make it possible to implement bug fixes and manual improvements if necessary.