Cfreptiles & Amphibians
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Economic and Ecological Implications of Shifting Cultivation in Mizoram, India Environmental Science and Engineering
Environmental Science Vishwambhar Prasad Sati Economic and Ecological Implications of Shifting Cultivation in Mizoram, India Environmental Science and Engineering Environmental Science Series Editors Ulrich Förstner, Technical University of Hamburg-Harburg, Hamburg, Germany Wim H. Rulkens, Department of Environmental Technology, Wageningen, The Netherlands Wim Salomons, Institute for Environmental Studies, University of Amsterdam, Haren, The Netherlands The protection of our environment is one of the most important challenges facing today’s society. At the focus of efforts to solve environmental problems are strategies to determine the actual damage, to manage problems in a viable manner, and to provide technical protection. Similar to the companion subseries Environmental Engineering, Environmental Science reports the newest results of research. The subjects covered include: air pollution; water and soil pollution; renaturation of rivers; lakes and wet areas; biological ecological; and geochemical evaluation of larger regions undergoing rehabilitation; avoidance of environmental damage. The newest research results are presented in concise presentations written in easy to understand language, ready to be put into practice. More information about this subseries at http://www.springer.com/series/3234 Vishwambhar Prasad Sati Economic and Ecological Implications of Shifting Cultivation in Mizoram, India 123 Vishwambhar Prasad Sati Department of Geography and Resource Management Mizoram University (A Central University) Aizawl, Mizoram, India ISSN 1863-5520 ISSN 1863-5539 (electronic) Environmental Science and Engineering ISSN 1431-6250 ISSN 2661-8222 (electronic) Environmental Science ISBN 978-3-030-36601-8 ISBN 978-3-030-36602-5 (eBook) https://doi.org/10.1007/978-3-030-36602-5 © Springer Nature Switzerland AG 2020 This work is subject to copyright. -

The Mizoram Gazette EXTRA ORDINARY Published Byauthority Regn
The Mizoram Gazette EXTRA ORDINARY Published byAuthority Regn. No. NE-313(MZ) 2006-2008 Rs. 2/- per issue VOL - XXXVII Aizawl, Thursday 11.9.2008 Bhadra 20, S.E. 1930, Issue No. 367 NOTIFICATION No. B. 14016/30/07-LADNC, the 9th September, 2008. LushaiHills District (Administration ofJustice) Rules, 1953, Sec 2 (1) (i) in thuneihna a pek angin Mizoram Governor chuan a hnuaia J... tarian ViUage Council te tan A}·rNEXURE a tarlan ang hian an Boundary a siam a. Tunhma lama heng Village Council te tana boundary 10 siam tawh te chu a thiat nghal ani. It Amaherawh chu heng ramri te hi a tul anga enfiah theih a ni ang. 1 CHA\VILUNGVILLA.GE COUNCIL BOUNDARY 2. TLUNGVEL VILLAGE COUNCIL BOUNDARY 3. DARLAWNG VILLAGE COUNCIL BOUNDARY 4. PHULMAWI VILLAGE COUNCIL BOUNDARY 5. THINGSULTHLIAH, rtllNGSULTLANGNUAM LEHSELINGJOINTVILLAGE COUNCIL BOUNDARY 6. KEPRAN VILLAGE COUNCIL BOUNDARY 7. SAWLENG VILLAGE COUNCIL BOUNDARY 8. N. SERZAWLVILLAGE COUNCIL BOUNDARY 9. SATEEKVILLAGE COUNCIL BOUNDARY 10. MAUBUANG VILLAGE COUNCIL BOUNDARY 11. LENCHIM VILLAGE COUNCIL BOUNDARY 12. RUALLUNG LEH RULCHAWM VILLAGE COUNCIL BOUNDARY 13. DAIDO VILLAGE COUNCIL BOUNDARY 14. N.E.TLANGNUAM VILLAGE COUNCIL BOUNDARY 15. PHUAIBUANG VILLAGE COUNCIL BOUNDARY 16. KHAWLIAN VILLAGE COUNCIl BOUNDARY 17. MUALLUNGTHU VILLAGE COlJNCIL BOUNDARY 18. KELSIH VILLAGE COUNCIL BOUNDARY 19. MELRIATVILLAGE COUNCIL BOUNDARY 20. HUALNGOHMUN VILLAGE COUNCIL BOUNDARY Ex-367/2008 - 2 - 21. SAMTLANG VILLAGE COUNCIL BOUNDARY 22. FALKAWN VILLAGE COUNCIL BOUNDARY 23. N.KHAWLEK VILLAGE COUNCIL BOUNDARY 24. VANBAWNG VILLAGE COUNCIL BOUNDARY 25. LAMHERH VILLAGE COUNCIL BOUNDARY 26. ZAWNGIN VILLAGE COUNCIL BOUNDARY 27. SUANGPUILAWN VILLAGE COUNCIL BOUNDARY 28. SAILAM VILLAGE COUNCIL BOUNDARY 29. -
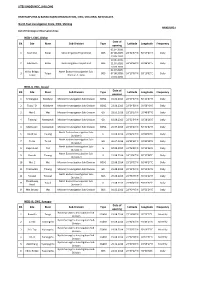
Hydrological Observation Sites
SITES UNDER NEIC, SHILLONG BRAHMAPUTRA & BARAK BASIN ORGANIZATION, CWC, SHILLONG, MEGHALAYA North‐East Investigation Circle, CWC, Shillong ANNEXURE‐I List of Hrdrological Observation Sites: NEID‐I, CWC, Silchar Date of SN. Site River Sub‐Division Type Latitude Longitude Frequency opening 02.07.2009, 1 Kashithal Sonai Sonai Irrigation Project Unit GDS 07.08.2009, 24°32'37''N 92°57'25''E Daily 01.05.2010 29.06.2009, 2 Kulicherra Rukni Rukni Irrigation Project Unit GDS 21.07.2009, 24°29'26''N 92°48'21''E Daily 01.05.2010 02.07.2009, Valley Bridge, North Eastern Investigation Sub‐ 3 Tuipui GDS 07.08.2009, 24°13'36''N 93°10'02''E Daily Tuipui Division‐5, Baite 01.05.2010 NEID‐II, CWC, Aizawl Date of SN. Site River Sub‐Division Type Latitude Longitude Frequency opening 1 Sihtlangpui Kolodyne Mizoram Investigation Sub‐Division GDSQ 01.01.2015 22°57'37''N 92°24'37''E Daily 2 Tuipui 'D' Kolodyne Mizoram Investigation Sub‐Division GDSQ 22.06.2013 22°54'50''N 93°56'40''E Daily 3 Mat‐1 Mat Mizoram Investigation Sub‐Division GD 23.09.2013 23°18'99''N 92°48'87''E Daily 4 Tlabung Karnaphuli Mizoram Investigation Sub‐Division GD 10.08.2013 22°53'54''N 92°28'26''E Daily 5 Mateswari Karnaphuli Mizoram Investigation Sub‐Division GDSQ 25.07.2013 22°56'25''N 92°31'32''E Daily North Eastern Investigation Sub‐ 6 Reiek Kai Tlawng G 07.08.2014 23°42'43''N 92°40'00''E Daily Division‐3 North Eastern Investigation Sub‐ 7 Turial Tuirial GD 30.07.2014 23°51'00''N 92°49'48''E Daily Division‐3 North Eastern Investigation Sub‐ 8 Dapchhuah Tut G 04.08.2014 23°46'09''N -

2020 Special Issue
Journal Home page : www.jeb.co.in « E-mail : [email protected] Original Research Journal of Environmental Biology TM p-ISSN: 0254-8704 e-ISSN: 2394-0379 JEB CODEN: JEBIDP DOI : http://doi.org/10.22438/jeb/4(SI)/MS_1903 Plagiarism Detector Grammarly Ichthyofauna of Dampa Tiger Reserve Rivers, Mizoram, North-Eastern India Lalramliana1*, M.C. Zirkunga1 and S. Lalronunga2 1Department of Zoology, Pachhunga University College, , Aizawl-796 001, India 2Systematics and Toxicology Laboratory, Department of Zoology, Mizoram University, Aizawl – 796 004, India *Corresponding Author Email : [email protected] Paper received: 04.02.2020 Revised received: 03.07.2020 Accepted: 10.07.2020 Abstract Aim: The present study was undertaken to assess the fish biodiversity in buffer zone of rivers of the Dampa Tiger Reserve, Mizoram, India and to evaluate whether the protected river area provides some benefits to riverine fish biodiversity. Methodology: Surveys were conducted in different Rivers including the buffer zone of Dampa Tiger Reserve during the period of November, 2013 to May, 2014 and October, 2019. Fishes were caught using different fishing nets and gears. Collected fish specimens were identified to the lowest possible taxon using taxonomic keys. Specimens were deposited to the Pachhunga University College Museum of Fishes (PUCMF) and some specimens to Zoological Survey of India (ZSI) Kolkata. Shannon-Wiener diversity index was calculated. Results: A total of 50 species belonging to 6 orders, 18 families and 34 genera were collected. The order Cypriniformes dominated the collections comprising 50% of the total fish species collected. The survey resulted in the description of 2 new fishOnline species, viz. -

Nansei Islands Biological Diversity Evaluation Project Report 1 Chapter 1
Introduction WWF Japan’s involvement with the Nansei Islands can be traced back to a request in 1982 by Prince Phillip, Duke of Edinburgh. The “World Conservation Strategy”, which was drafted at the time through a collaborative effort by the WWF’s network, the International Union for Conservation of Nature (IUCN), and the United Nations Environment Programme (UNEP), posed the notion that the problems affecting environments were problems that had global implications. Furthermore, the findings presented offered information on precious environments extant throughout the globe and where they were distributed, thereby providing an impetus for people to think about issues relevant to humankind’s harmonious existence with the rest of nature. One of the precious natural environments for Japan given in the “World Conservation Strategy” was the Nansei Islands. The Duke of Edinburgh, who was the President of the WWF at the time (now President Emeritus), naturally sought to promote acts of conservation by those who could see them through most effectively, i.e. pertinent conservation parties in the area, a mandate which naturally fell on the shoulders of WWF Japan with regard to nature conservation activities concerning the Nansei Islands. This marked the beginning of the Nansei Islands initiative of WWF Japan, and ever since, WWF Japan has not only consistently performed globally-relevant environmental studies of particular areas within the Nansei Islands during the 1980’s and 1990’s, but has put pressure on the national and local governments to use the findings of those studies in public policy. Unfortunately, like many other places throughout the world, the deterioration of the natural environments in the Nansei Islands has yet to stop. -

Pseudolaguvia Virgulata, from Mizoram, India
http://sciencevision.info Sci Vis 10 (2), 73 Research Report April-June, 2010 ISSN 0975-6175 On the new catfish, Pseudolaguvia virgulata, from Mizoram, India Lalramliana Department of Zoology, Pachhunga University College, Mizoram University, Aizawl 796001, India A new species of catfish was recently identi- fied from some major rivers of Mizoram. Heok Hee Ng and Lalramliana named the new catfish Pseudolaguvia virgulata, after its distinctively striped colour pattern (virgulata = “striped” in Latin). Besides its distinctive colour pattern, which consists of pale stripes running along the entire length of the body, a pale y-shaped marking on the head and brown stripes running through the caudal fin lobes, the new catfish also differs from congeners in other characters. These in- clude: head width 21.2–24.4% standard length; pectoral-fin length 28.5–29.1% standard length; length of dorsal-fin base 17.2–19.9% standard length; dorsal-spine length 21.5–24.0% standard length; serrated anterior edge of dorsal spine; thoracic adhesive apparatus reaching beyond base of last pectoral-fin ray; body depth at anus 14.5–17.4% standard length; length of adipose- fin base 12.9–15.0% standard length; caudal peduncle length 18.2–20.2% standard length; caudal peduncle depth 7.8–9.7 % standard length; snout length 48.0–54.9% head length; interorbital distance 29.3–35.2% head length; 29 –30 vertebrae. Pseudolaguvia virgulata was collected from river system, one of the three rivers that form clear, shallow, moderately flowing streams with the Ganges Delta. a predominantly sandy bottom. -

Cfreptiles & Amphibians
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL & AMPHIBIANS15, NO 4 • DEC 2008 • 28(2):189 270–273 • AUG 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FirstFEATURE ARTICLESRecord of Interspecific Amplexus . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: betweenOn the Road to Understandinga Himalayan the Ecology and Conservation of the Toad, Midwest’s Giant Serpent Duttaphrynus ...................... Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: himalayanusA Hypothetical Excursion ............................................................................................................................ (Bufonidae), and a RobertHimalayan W. Henderson 198 RESEARCH ARTICLES Paa. TheFrog, Texas Horned Lizard Nanorana in Central and Western Texas ....................... vicina Emily Henry, Jason(Dicroglossidae), Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida from ............................................. the BrianWestern J. Camposano, Kenneth L. Krysko, Himalaya Kevin M. Enge, Ellen M. Donlan, andof Michael India Granatosky 212 CONSERVATION ALERT . World’s Mammals in Crisis ...............................................................................................................................V. Jithin, Sanul Kumar, and Abhijit Das .............................. 220 . More Than Mammals ..................................................................................................................................................................... -

Reproductive Biology of the Assam Forest Frog, Hydrophylax Leptoglossa
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & IRCFAMPHIBIANS REPTILES • VOL 15,& NAMPHIBIANSO 4 • DEC 2008 •189 25(2):139–141 • AUG 2018 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATURE ARTICLES Reproductive. Chasing Bullsnakes (Pituophis catenifer Biology sayi) in Wisconsin: of the Assam Forest On the Road to Understanding the Ecology and Conservation of the Midwest’s Giant Serpent ...................... Joshua M. Kapfer 190 Frog,. The SharedHydrophylax History of Treeboas (Corallus grenadensis) and leptoglossaHumans on Grenada: (Cope 1868) A Hypothetical Excursion ............................................................................................................................Robert W. Henderson 198 RESEARCH(Anura: ARTICLES Ranidae), from Lawachara . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................NationalBrian J. Camposano, Park, Kenneth L. Krysko, Kevin Bangladesh M. Enge, Ellen M. Donlan, and Michael Granatosky 212 CONSERVATIONMd. Mokhlesur ALERT Rahman, Md. Fazle Rabbe, and Md. Mahabub Alam . World’s Mammals in Crisis ............................................................................................................................................................. 220 . More ThanDepartment Mammals of.............................................................................................................................. -

Development of Edna Assays for Three Frogs
Development of eDNA assays for monitoring three endangered frog species (Litoria dayi, L. lorica and L. nannotis) in Australia’s wet tropics Report by Richard C. Edmunds, Cecilia Villacorta-Rath, Roger Huerlimann and Damien Burrows © James Cook University, 2019 Development of eDNA assays for monitoring three endangered frog species (Litoria dayi, L. lorica and L. nannotis) in Australia's wet tropics is licensed by James Cook University for use under a Creative Commons Attribution 4.0 Australia licence. For licence conditions see creativecommons.org/licenses/by/4.0 This report should be cited as: Edmunds, R.C., Villacorta-Rath, C., Huerlimann, R., and Burrows, D. 2019. Development of eDNA assays for monitoring three endangered frog species (Litoria dayi, L. lorica and L. nannotis) in Australia's wet tropics. Report 19/24, Centre for Tropical Water and Aquatic Ecosystem Research (TropWATER), James Cook University Press, Townsville. Cover photographs Front cover: Litoria dayi (photo Trent Townsend/Shutterstock.com). Back cover: Litoria lorica (left) and L. nannotis (right) in situ (photo: Conrad Hoskin). This report is available for download from the Northern Australia Environmental Resources (NAER) Hub website at nespnorthern.edu.au The Hub is supported through funding from the Australian Government’s National Environmental Science Program (NESP). The NESP NAER Hub is hosted by Charles Darwin University. ISBN 978-1-925800-33-3 June, 2019 Printed by Uniprint Contents Acronyms....................................................................................................................................iv -

Final Report
Assessing the distribution and conservation status of Variable Bush Frog (Raorchestes chalazodes Gunther, 1876) in the Konni Bio-reserve – Shenduruni Wildlife Sanctury (Konni Forest Division, Achankovil Forest Divison , Thenmala Forest Divison and Shenduruni Wildlife Division) of Western Ghats, India Final report (2014-2015) David V Raju Manoj P Tropical Institute of Ecological Sciences Kottayam, Kerala Summary Amphibians and their tadpoles are significant in the maintenance of ecosystems, playing a crucial role as secondary consumers in the food chain, nutritional cycle, and pest control. Over the past two decades, amphibian research has gained global attention due to the drastic decline in their populations due to various natural and anthropogenic causes. Several new taxa have been discovered during this period, including in the Western Ghats and Northeast regions of Indian subcontinent. In this backdrop, the detailed account on the population and conservation status of Raorchestes chalazodes, a Rhacophorid frog which was rediscovered after a time span of 136 years was studied in detail. Forests of Konni bio reserve - Shenduruni wildlife sanctuary were selected as the study area and recorded the population and breeding behavior of the critically endangered frog. Introduction India, which is one of the top biodiversity hotspots of the world, harbors a significant percentage of global biodiversity. Its diverse habitats and climatic conditions are vital for sustaining this rich diversity. India also ranks high in harboring rich amphibian diversity. The country, ironically also holds second place in Asia, in having the most number of threatened amphibian species with close to 25% facing possible extinction (IUCN, 2009). The most recent IUCN assessments have highlighted amphibians as among the most threatened vertebrates globally, with nearly one third (30%) of the world’s species being threatened (Hof et al., 2011). -

2. Executive Summary of Revised Action Plan for 9 Rivers in Mizoram
EXECUTIVE SUMMARY OF THE ACTION PLAN FOR CONSERVATION OF NINE RIVERS IN MIZORAM, 2019 In compliance of the orders of the Hon’ble NGT on OA No. 673/2018 dt. 20.09.2018, 19.12.2018 & 08.04.2019 Prepared by RIVER REJUVENATION COMMITTEE (RRC) GOVT. OF MIZORAM Contents Page 1. Introduction 1 2. Summary of Action Plan of Tiau River 5 3. Summary of Action Plan of Tlawng River 7 4. Summary of Action Plan of Tuipui River 9 5. Summary of Action Plan of Tuivawl River 11 6. Summary of Action Plan of Chite Stream 13 7. Summary of Action Plan of Mat River 15 8. Summary of Action Plan of Saikah Stream 17 9. Summary of Action Plan of Tuikual River 19 10. Summary of Action Plan of Tuirial River 21 11. Abstract of the financial requirement 23 EXECUTIVE SUMMARY OF THE ACTION PLAN FOR THE NINE(9) RIVERS OF MIZORAM In compliance to the orders of the Hon’ble NGT dated 20.09.2018, 19.12.2018 & 08.04.2019 in the matter of OA No. 673/2018 - M.C Mehta-Vrs-Union of India & Ors related to the News item dated 17.09.2018, published in ‘‘The Hindu” under the heading “More river stretches are now critically polluted ”, River Rejuvenation Committee (RRC), Govt. of Mizoram has prepared Action Plan for conservation of nine(9) rivers in Mizoram, which are identified to be polluted by CPCB based on BOD level during 2016 and 2017. The nine (9) identified polluted rivers are : i) 1 river (Tiau) - Priority III ii) 3 rivers (Tlawng, Tuipui and Tuivawl) - Priority IV iii) 5 rivers (Chite, Mat, Saikah, Tuikual and Tuirial)- Priority V The Action Plan is prepared for conservation, rather than rejuvenation of the rivers since the identified 9 polluted river stretches in Mizoram are already within the prescribed limits of BOD (Data annexed), preparation of action plan for rejuvenation of these rivers for bringing down the BOD level does not arise for these rivers. -

Cathelicidin-OA1, a Novel Antioxidant Peptide Identified from An
www.nature.com/scientificreports OPEN Cathelicidin-OA1, a novel antioxidant peptide identifed from an amphibian, accelerates skin Received: 9 October 2017 Accepted: 2 January 2018 wound healing Published: xx xx xxxx Xiaoqing Cao1, Ying Wang2, Chunyun Wu3, Xiaojie Li4, Zhe Fu3, Meifeng Yang3, Wenxin Bian3, Siyuan Wang2, Yongli Song3, Jing Tang4 & Xinwang Yang3 Cathelicidins play pivotal roles in host defense. The discovery of novel cathelicidins is important research; however, despite the identifcation of many cathelicidins in vertebrates, few have been reported in amphibians. Here we identifed a novel cathelicidin (named cathelicidin-OA1) from the skin of an amphibian species, Odorrana andersonii. Produced by posttranslational processing of a 198-residue prepropeptide, cathelicidin-OA1 presented an amino acid sequence of ‘IGRDPTWSHLAASCLKCIFDDLPKTHN′ and a molecular mass of 3038.5 Da. Functional analysis showed that, unlike other cathelicidins, cathelicidin-OA1 demonstrated no direct microbe-killing, acute toxicity and hemolytic activity, but did exhibit antioxidant activity. Importantly, cathelicidin-OA1 accelerated wound healing against human keratinocytes (HaCaT) and skin fbroblasts (HSF) in both time- and dose-dependent manners. Notably, cathelicidin-OA1 also showed wound-healing promotion in a mouse model with full-thickness skin wounds, accelerating re-epithelialization and granulation tissue formation by enhancing the recruitment of macrophages to the wound site, inducing HaCaT cell proliferation and HSF cell migration. This is the frst cathelicidin identifed from an amphibian that shows potent wound-healing activity. These results will help in the development of new types of wound-healing agents and in our understanding of the biological functions of cathelicidins. Cathelicidins, which belong to a group of cationic peptides with amphipathic properties, play critical roles in host defense1.