1997 AAZV Proceedings.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cheyenne Mountain Zoo
Cheyenne Mountain Zoo Particulars Organisation Name Cheyenne Mountain Zoo Corporate Website Address cmzoo.org Primary Activity or Product Environmental NGO Related Company(ies) None Country Operations United States Membership Number 6-0017-10-000-00 Membership Type Ordinary Members Membership Category Environmental and Conservation NGOs Particulars ACOP 2013/2014 - Cheyenne Mountain Zoo Environmental and Conservation NGOs Operational Profile 1.1 What are the main activities of your organization ? Cheyenne Mountain Zoo's main acitivites are wildlife conservation, captive breeding, and education. Our mission statement: A leader in conservation, captive breeding, and animal care, Cheyenne Mountain Zoo connects people to wildlife and wild places through experiences that inspire action. 1.2 Does your organization use and/or sell any palm oil? Yes 1.3 Activities undertaken to promote sustainable palm oil, the RSPO and/or members in the reporting period The Cheyenne Mountain Zoo continues to take a lead in our industry in the U.S. on palm oil sustainability. Our palm oil awareness program has made it possible to educate our over 600,000 guests as well as other zoos about the importance of using only sustainable palm oil. We promoted the RSPO and sustainable palm oil in the following ways: 1. We hosted the first ever sustainable palm oil symposium at Cheyenne Mountain Zoo (CMZ) in April 2014. Representatives from many zoos attended, as well as guest speakers from the RSPO and other RSPO E-NGO representatives. 2. CMZ also hosted an AZA (Association of Zoos and Aquariums) Palm Oil Task Force meeting at which we created a palm oil position statement. -

Annual Report Table of Contents
May 2020 - April 2021 ANNUAL REPORT TABLE OF CONTENTS Who We Are . 3 The Year’s Highlights . 4 New Zoo Family Members . 7 Conservation . 8 EdVenture . 11 Numbers at a Glance . 12 Financial Summary . 15 Donors & Sponsors . 16 1926 Society . 19 Who We Are OUR MISSION A leader in conservation, captive breeding and animal care, Cheyenne Mountain Zoo connects people with wildlife and wild places through experiences that inspire action. OUR Every Kid . Every Time . Goosebumps! VISION Every kid, of any age, will have an experience for a lifetime with every visit. OUR With only a mission and vision to guide them, these are LEADERS the people who volunteer their time to make sure the greatness of Cheyenne Mountain Zoo continues. 2020 - 2021 BOARD OF DIRECTORS Officers Hans Mueh, Chair Tia Ferguson, Vice Chair Vic Andrews, Treasurer Ann Naughton, Secretary Bob Chastain, President & CEO Directors Ed Anderson Ken Keene JL Austgen Carol Kleiner Amy Bales Kevin Kratt Matt Carpenter Trevor Miller Mike Edmonds Susan Sallee Peri Faricy Mari Sinton-Martinez Stephannie Fortune Sue Switzer Lynn Janeczek Sally Veitch Susan Johnson Brenda Whitlock Barbara Kalbli Gary Winegar Honorary Director Katherine H. Loo May 2020 - April 2021 CHEYENNE MOUNTAIN ZOO 3 The Year’s Highlights Q4C HITS $3 MILLION MILESTONE DEMOLITION OF MONKEY PAVILION OPENING OF WATER’S EDGE: AFRICA Every visit to CMZoo is conservation in In September 2020, we announced plans to action. In July 2020, CMZoo and its guests and demolish Monkey Pavilion. Built in 1942, it members celebrated a huge milestone, having provided good homes for its residents, but raised $3 million since the Zoo’s Quarters for fell short of supporting our mission to connect Conservation (Q4C) program launched in guests with animals and inspire them to protect 2008. -

Cmzoo Internship Application Packet
Cheyenne Mountain Zoo Animal Department Program Internship Position Description Internship Fee: $505.00 / $305.00 Department: Animal Area Principal Function: To learn how to provide the highest quality care and husbandry of the animals in the area to which they are assigned. To learn how to educate, entertain and provide exemplary guest service experiences to all zoo guests through various programs, activities and continuous guest interaction. Fee: Since we approach our internships as enrollment into a learning institution, certain fees apply. The cost of an internship is $505.00 between March 1st and August 31st . Internships are offered at a discounted rate at $305.00 between September 1st and February 28th. This includes a zoo membership that is valid through the duration of your internship, and includes all the benefits that come with the membership (e.g.: free admission to the zoo on your days off, discounts on zoo programs, etc.) Please consult your career counselor at your school to inquire about receiving academic credit for your internship. Supervision: Reports to Animal Keepers in the Area interns are assigned. Animal Keepers report to Animal Supervisor. Major Responsibilities: • Each internship consists of 360 hours for each semester. These hours MUST be completed within the season for which the student is registered for that specific internship. Incomplete hours will reflect in final grade. • Each intern works a minimum of 3 days per week • Punctuality- Must be on time to assigned shift and location • Intern will be in uniform and wear a nametag while on Zoo grounds. Appropriate footwear is required. Appropriate outerwear is required for all the weather conditions: rain, snow, wind, hail, etc. -

President's Message As We Reflect on the Year Behind Us, We Cannot Help but Think About the Exciting Projects in Our Immediate Future
PULL-OUT SUPPLEMENT President's Message As we reflect on the year behind us, we cannot help but think about the exciting projects in our immediate future. At Cheyenne Mountain Zoo, our passion is to ensure those unique, memorable and once-in-a-lifetime experiences for each and every person that enters our front gate. In this past year, we added a new entry plaza as well as a completely redesigned Grizzly Grill—improvements specifically selected to enhance and strengthen the guest experience. At Cheyenne Mountain Zoo, we are building for the future. We are building for you, the community of Colorado Springs. We cannot take our popularity for granted and refuse to let complacency set in. We are extremely aware of the importance of ongoing improvements—to the facilities themselves, to the programs offered, to the customer service provided and to the value afforded Zoo members. Our staff works tirelessly each day to continue the wonderful tradition that is Cheyenne Mountain Zoo. We recognize the invaluable support of our donors, volunteers and sponsors. Their assistance grants us the opportunity to dream…and dream big. So, we thank you. Bob Chastain President & CEO Our Mission Our Vision A leader in conservation, captive breeding and animal care, Cheyenne Mountain Zoo connects Every Kid, Every Time, Goosebumps! people with wildlife and wild places through Every kid, of any age, will have an experiences that inspire action. experience for a lifetime with every visit. 33754_CMZ AnnualReport_09-10a_R2.indd 1 8/3/10 8:41 AM In another conservation effort on the other side of the world, the Zoo’s Animal We Couldn't Do It Without You! Behavior Manager, Megan Sanders, traveled to Mongolia in August 2009 to work Thanks to our friends and with the Snow Leopard Trust. -

Loevy Family History-002-Archibald Williams
THOMAS E. CRONIN AND ROBERT D. LOEVY TOM CRONIN AND BOB LOEVY IN THE NEWSPAPERS 2019 IN THE NEWSPAPERS – 2019 Page 1 THOMAS E. CRONIN AND ROBERT D. LOEVY IN THE NEWSPAPERS – 2019 Page 2 THOMAS E. CRONIN AND ROBERT D. LOEVY INTRODUCTION In the fall of 2016 two professors of Political Science at Colorado College, Thomas E. Cronin and Robert D. Loevy, were offered the opportunity to write periodic opinion columns for the local newspaper – the Colorado Springs Gazette. This launched a longtime project of the two professors writing for the newspaper for a number of years. Previously Tom Cronin and Bob Loevy had written together for the Denver Post, but only periodically. They also collaborated on a book on government and politics in Colorado. This book is a collection of the newspaper stories Cronin and Loevy wrote for the Colorado Springs Gazette in the year 2019. These are the stories as Cronin and Loevy wrote them. The dates on the stories are when the columns were written and not when they appeared in the newspaper. The headlines are the “working” headlines used by Cronin and Loevy and not the headlines used in the newspaper. This book offers the opportunity to read the facts, ideas, and opinions of two scholars of Colorado politics all in one place for the calendar year 2019. The actual published versions of these articles can be found on the Denver Post or the Colorado Springs Gazette websites. Except for the headlines, most of the articles were published exactly the way that Cronin and Loevy wrote them. -

The Broadmoor Soaring Adventure
WELCOME TO THE BRO ADMOOR Our Broadmoor family would like to extend a warm welcome to you and your family as you have chosen a magnificent destination to share with your loved ones. In 1918, Spencer and Julie Penrose saw their dream become a reality as their sanctuary for respite and relaxation was completed at the base of Cheyenne Mountain. Today, we are fortunate enough to have expanded our oerings to include a wide variety of outdoor activities and Wilderness Adventures for our guests. As we celebrate over a “Century of Service” with a sta that hails from all corners of the world, it is our privilege to continue the tradition of making your stay as satisfying, enjoyable, and memorable as possible. Within this directory, you will find the services and amenities which are readily available to you during your visit. Please enjoy your stay. Stephen Bartolin, Jr. Chairman Jack Damioli President and CEO Ann M. Alba Vice President and Resident Manager WELCOME THE BROADMOOR SOARING ADVENTURE THE BROADMOOR SEVEN FALLS THE BROADMOOR CLOUD CAMP THE BROADMOOR THE BROADMOOR PIKES PEAK COG RAILWAY FLY FISHING CAMP DMOOR WILDERNESS EXPERIENCE A THE BRO THE BROADMOOR RANCH AT EMERALD VALLEY THE BROADMOOR WILDERNESS EXPERIENCES AND ADVENTURES THE BROADMOOR CLOUD CAMP Located 3,000 feet above the resort with 360-degree views is Cloud Camp. Its accolades include Top 10 Resort Hotels in the American West from Travel+Leisure, Best Room with a View for its unique “Fire Tower Suite” from Sunset Magazine Travel Awards, and Top 10 Clifftop Retreats Around the World from Architectural Digest. -

Small Carnivore CAMP 1993.Pdf
SMALL CARNIVORE CONSERVATION ASSESSMENT AND MANAGEMENT PLAN Final Review Draft Report 1G May 1994 Edited and compiled by Roland Wirth, Angela Glatston, Onnie Byers, Susie Ellis, Pat Foster-Turley, Paul Robinson, Harry Van Rompaey, Don Moore, Ajith Kumar, Roland Melisch, and Ulysses Seal Prepared by the participants of a workshop held in Rotterdam, The Netherlands 11-14 February 1993 A Collaborative Workshop IUCN/SSC MUSTELID, VIVERRID, AND PROCYONID SPECIALIST GROUP IUCN/SSC OTTER SPECIALIST GROUP IUCN/SSC CAPTIVE BREEDING SPECIALIST GROUP Sponsored by The Rotterdam Zoo IUCN/SSC Sir Peter Scott Fund United Kingdom Small Carnivore Taxon Advisory Group A contribution of the IUCN/SSC Captive Breeding Specialist Group, IUCN/SSC Mustelid, Viverrid, and Procyonid Specialist Group and the IUCN/SSC Otter Specialist Group. The Primary Sponsors of the Workshop were: The Rotterdam Zoo, IUCN/SSC Peter Scott Fund, United Kingdom Small Carnivore Taxon Advisory Group. Cover Photo: Malayan Civet, Viverra tangalunga by Roland Wirth. Wirth, R., A Glatston, 0. Byers, S. Ellis, P. Foster-Turley, P. Robinson, H. Van Rompaey, D. Moore, A Kumar, R. Melisch, U.Seal. (eds.). 1994. Small Carnivore Conservation Assessment and Management Plan. IUCN/SSC Captive Breeding Specialist Group: Apple Valley, MN. Additional copies of this publication can be ordered through the IUCN/SSC Captive Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124. Send checks for US $35.00 (for printing and shipping costs) payable to CBSG; checks must be drawn on a US Bank. Funds may be wired to First Bank NA ABA No. 091000022, for credit to CBSG Account No. -

Protection of Endangered Gorillas and Chimpanzees in International Trade: Can CITES Help Valerie Karno
Hastings International and Comparative Law Review Volume 14 | Number 4 Article 11 1-1-1991 Protection of Endangered Gorillas and Chimpanzees in International Trade: Can CITES Help Valerie Karno Follow this and additional works at: https://repository.uchastings.edu/ hastings_international_comparative_law_review Part of the Comparative and Foreign Law Commons, and the International Law Commons Recommended Citation Valerie Karno, Protection of Endangered Gorillas and Chimpanzees in International Trade: Can CITES Help, 14 Hastings Int'l & Comp. L. Rev. 989 (1991). Available at: https://repository.uchastings.edu/hastings_international_comparative_law_review/vol14/iss4/11 This Note is brought to you for free and open access by the Law Journals at UC Hastings Scholarship Repository. It has been accepted for inclusion in Hastings International and Comparative Law Review by an authorized editor of UC Hastings Scholarship Repository. For more information, please contact [email protected]. Protection of Endangered Gorillas and Chimpanzees in International Trade: Can CITES Help? By VALERIE KARNO* Member of the Class of 1991 I. INTRODUCION At least forty thousand primates are traded internationally every year.' Of these, at least thirteen thousand are sold illegally.2 Smugglers are currently trading live, endangered primates throughout the world,3 and statistics indicate that unless this trade is stopped, certain primates, such as the gorilla and wild chimpanzee, will shortly be extinct.4 Over the last twenty years, commerce practices have caused between forty and ninety thousand needless chimpanzee deaths.5 The effect of these deaths on the viability of the species is profound. Field surveys estimate the current world chimpanzee population at less than 250,000 individuals. -
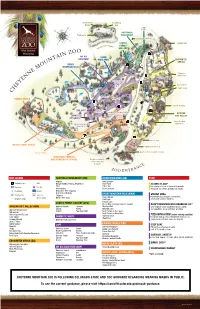
Cheyenne Mountain Zoo Is Following Colorado State and Cdc Guidance Regarding Wearing Masks in Public
Mountaineer Bouldering Market Wall Budgie Buddies AUSTRALIA Playground WALKABOUT DRIVE TO THE SHRINE OF THE S U N SCUTES Elephant Barn EER SUM FAMILY TAIN MIT UN MO GALLERY MY BIG ASIAN BACKYARD HIGHLANDS ENCOUNTER AFRICA ROCKY MOUNTAIN WILD Admin Building Elephant Demo Area Chicken Feeding Experience Elson’s Place CONSTRUCTION Safari Lodge AREA N U S E H T F O Old Gnarly E Grizzly Grill N PRIMATE WORLD I R H S Carousel E H T O T Giraffe Building E V I R THE LOFT Lodge at D Moose Lake Pizza with Carousel Sweets a View AFRICAN DOMESTIC ROCKY RIFT VALLEY GOATS Cozy Goat CLIFFS Giraffe Feeding Rocky Mountain Wild Experience Barbecue Co. Mountaineer Sky Ride Mountain Goat Activity Yard D Stroller Hut ICE ROA SERV Safari Cabin WATER’S EDGE: AFRICA Thundergod Gift Shop Penguin Beach Quarters for Conservation Kiosks EDVENTURE COMPLEX AND MEMBERSHIP OFFICES Entrance to road to Shrine of the Sun (5 m.p.h.) MAP LEGEND AUSTRALIA WALKABOUT (AW) ASIAN HIGHLANDS (AH) FOOD Alligator Amur Leopard Sidewalk Locator ATM Budgie Buddies Feeding Experience Amur Tiger ELSON’S PLACE* Emu Pallas’ Cat Restrooms First Aid Refreshing selection of flavored homemade Invertebrates Snow Leopard lemonade, ice cream, pretzels and snacks. Food/Drinks Elevator Matschie’s Tree Kangaroo Red-Necked Wallaby ROCKY MOUNTAIN WILD (RMW) GRIZZLY GRILL Smoking Area Top of Zoo Shelduck Handmade pizza, burgers, sandwiches, (no foot traffic) White’s Tree Frog Alaska Moose Drinking Fountain Bald Eagle salads and seasonal favorites. Canada Lynx SCUTES FAMILY GALLERY (SFG) Grizzly Bear (via tower stairs or elevator) ROCKY MOUNTAIN WILD BARBECUE CO.* AFRICAN RIFT VALLEY (ARV) Burmese Python Tortoises Mexican Wolf Chef-inspired house-smoked barbecue, salads Lizards Turtles Mountain Lion African Crowned Crane and appetizers. -

2018 OCP Annual Report
2018 ANNUAL REPORT Project Staff A Letter From John President (US) – John Lukas On-site Director – Rosmarie Ruf OKAPI The Okapi Conservation Project around the Reserve, we invest in Accountant – Mutahinga Mumbere Eleme CONSERVATION Asst. Accountant – Kambale Katsuva PROJECT since its inception has been all projects that improve their quality Julien about investment. We invest in the of life. We continued to invest in Program Officer (US) – Lucas Meers place where okapi live, invest in the bringing on board talented young Education Coordinator – M’monga Kiete communities that share the lands people, especially women. We Agroforestry Enckoto Bameseto Mission with okapi, invest in people that also invested in the education of Makubuli Mwanika TO CONSERVE THE OKAPI IN THE WILD, WHILE drive program success and invest in the three children of our educator, Masiyiri Mulawa PRESERVING THE BIOLOGICAL AND CULTURAL fostering a good working relationship Kalinda, who was killed in the attack Mpinda Tchinkunku DIVERSITY OF THE ITURI FOREST Muvi Yalala with government officials that on our vehicle. Muhindo Muliwavyo Kasereka Tsongo oversee conservation in DRC. Sambi Mukandilwa It has been, and continues to Lobo Lina In 2018 amidst regional instability, be, our strategy to invest in all Nadepa Awelekyalanga Manbgeto Bambakonda Therese our staff had to deal with the stakeholders involved in or Educators unpredictable events that led to impacted by the conservation of Gomo Akya an ambush attack on an OCP okapi habitat. We have seen our Kasereka Kyove Mumbere Kayenga vehicle where six innocent people holistic investment approach (which Toliba Maseko died, the spread of the Ebola is made possible by your donations Carine MAKONGA epidemic closer to the Reserve, and grants) pay dividends in the Roger OZANDE Eric SIVINAVA more miners invading the Reserve form of over 8,000 square miles of KAMBALE MASTAKI Faustin Mbuza and uncertainty leading up to the intact okapi habitat protected that elections in December. -

2019 Downtown Partnership Annual Report
DOWNTOWN COLORADO SPRINGS 2019 ANNUAL REPORT TO THE COMMUNITY Downtown Partnership Downtown Ventures & PikeRide Downtown Development Authority Greater Downtown Colorado Springs Business Improvement District Downtown Partnership Staff Susan Edmondson President & CEO Laurel Prud’homme Vice President of Communications Tim Archer Public Space Manager Alexander Armani-Munn Economic Development Specialist Margo Baker Administrative & Member- ship Coordinator Katy Hartshorn Marketing Coordinator Len Kendall Director of Planning and Mobility To our Downtown Stakeholders, Claire Swinford Throughout 2019, Downtown Colorado Springs continued its rapid pace of Director of Urban transformation, welcoming new businesses, new residents and visitors while Engagement simultaneously devoting efforts to delivering new anchor attractions and Ana Valdez services for our bright future. It’s an exciting era for our city center, and Finance Officer Downtown Partnership is leading the way. Meeks Canine Companions for Place-based economic development delivers the whole package: fostering a Independence Trainee safe, connected, walkable urban environment; providing an exceptional experi- ence for shoppers, diners and cultural patrons; welcoming urban dwellers, new businesses and entrepreneurs; telling our story through robust marketing and PikeRide Staff social media channels; fostering an environment attractive to investment and innovation; and championing a city center that is the essential heartbeat to a Jolie NeSmith thriving region. Executive Director Brent Wegscheid To operate at our most efficient, we at Downtown Partnership know we are Operations Director better together. The Partnership serves as the management company for the Tyna Murray Downtown Development Authority and the Greater Downtown Colorado Administrative & Customer Springs Business Improvement District, and we leverage our charitable non- Service Manager profit arm, Downtown Ventures, for our urban engagement programs. -

2014-2015 Annual Report
May 2014 - April 2015 Annual Report President’s Message Table of Content Our Mission . 4 Our Vision . 4 Dear friends, supporters and donors, Our Leaders . 4 They say time flies when you’re having fun. We did have fun this past year, but I believe the year flew by because A Record Year of Support . 6 we were working hard toward our goals of providing exceptional animal care, offering once-in-a-lifetime guest interactions and saving imperiled wildlife. A New Australia Walkabout . 8 In our 2014 – 2015 fiscal year, after decades of hard work, the Zoo broke all-time attendance records, stabilizing the Zoo’s financial position and securing a place in the community as the most-attended attraction in Colorado New Members of Our Zoo Family . 10 Springs. Saving Imperiled Species One Quarter at a Time . 14 We started this past year with strong attendance numbers. In August, things got even better when we were named the 5th best zoo in the country and the 13th best zoo in the world by TripAdvisor. The attention allowed the Commitment to Conservation . 16 mission of the Zoo to flourish. Connecting Our Guests with Animals . 18 We improved exhibit spaces, animal training areas and our animal care programs. All of that great care resulted in healthy animals and several new babies. We also gave guests unprecedented access to our animals when we EdVenture for All . 20 started an elephant and rhino feeding program. The program afforded us the opportunity to donate $25,000 to the protection and conservation of wild elephants and rhinos, both of which are being killed at alarming rates.