New Records of Lamellate Mushrooms Associated with Sal from Shiwaliks, India Jitender Kumar and N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lockerbie Wildlife Trust Eskrigg Reserve August 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: SC 005538 August 2016 News Bulletin 1. View of pond on the 16th of August. 2. Confirmed wildlife sightings at the Reserve in August. a. Birds Blackbird, Black East Indian Duck, Blue Tit, Buzzard, Carrion Crow, Chaffinch, Coal Tit, Dunnock, Goldcrest, Goldfinch, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kingfisher, Little Grebe, Mallard, Moorhen, Nuthatch, Pheasant, Pied Flycatcher, Raven, Robin, Rook, Siskin, Song Thrush, Swallow, Treecreeper, Wood Pigeon, Wren. b. Mammals Bank Vole, Common Shrew, Fox, Hedgehog, Mole, Rabbit, Red Squirrel, Woodmouse. c. Reptiles and Amphibians Common Shrew - PC Hedgehog Common Lizard, Frog, Toad. d. Invertebrates Black Slug; Tree and White-tailed Bumble Bees; Common Carder Bee; Green-veined White, Large White, Meadow Brown, Painted Lady, Peacock, Red Admiral, Ringlet, Small Tortoiseshell and Small White butterflies; Crane-flies; Azure, Blue-tailed and Common Blue Damselflies; Common Darter, Common Hawker, Golden Ringed and Southern Hawker dragonflies; Froghoppers, Grasshoppers; Hoverflies; 7-Spot, 10-Spot and Larch Ladybirds; Midges; Mosquitoes; Common White Wave, Crescent, July Highflyer, LargeYellow Underwing and Silver-Y moths. 10-spot Ladybird Photographs: Jim Rae, Pam Copeland (PC). 1!! ! 3. August Photo-gallery. Row 1: Creeping Cinquefoil, Red Admiral Butterfly, Monkeyflower. Row 2: Peacock Butterfly, Fruiting Honeysuckle, Painted Lady Butterfly. Row 3: Dyer's Mazegill, Small Torytoiseshell Butterfly, Turkeytail. Row 4: Young Common Lizard, Common Carder Bee on Devil's-bit Scabious, Young Frog in reed-grass. Row 5: Burgundydrop Bonnet, Spider (Araneus diadematus), Yellow Stag's Horn. -

Plectological and Molecular Identification Of
Bangladesh J. Plant Taxon. 27(1): 67‒77, 2020 (June) © 2020 Bangladesh Association of Plant Taxonomists PLECTOLOGICAL AND MOLECULAR IDENTIFICATION OF ECONOMICALLY IMPORTANT WILD RUSSULALES MUSHROOMS FROM PAKISTAN AND THEIR ANTIFUNGAL POTENTIAL AGAINST FOOD PATHOGENIC FUNGUS ASPERGILLUS NIGER 1 SAMINA SARWAR*, TANZEELA AZIZ, MUHAMMAD HANIF , SOBIA ILYAS, 2 3 MALKA SABA , SANA KHALID AND MUHAMMAD FIAZ Department of Botany, Lahore College for Women University, Lahore, Pakistan Keywords: Aseptate; Biocontrol; Macrofungi; Micromycetes; Mycochemicals. Abstract Present study deals with the plectological and molecular analysis as well as use of economically important wild Russuloid mushrooms against food pathogenic fungus Aspergillus niger. Three different species of mushrooms viz., Russla laeta, R. nobilis, and R. nigricans were collected and identified from Himalayan range of Pakistan and are found as new records for this country. Major objective of this study was to highlight the importance of these wild creatures as antifungal agents against A. niger. For this purpose methanolic extract of selected mushrooms of different concentration levels viz., 1, 1.5, 2 and 3% were used. This activity is also first time reported from Pakistan by using this group of mushrooms. Results showed that all tested mushrooms exhibit growth inhibition of A. niger and can be used as biocontrol agents. R. nigricans showed maximum inhibition of fungus growth that is 62% at 3% concentrations while minimum inhibition was observed in R. nobilis at same concentration that is 43.6%. Introduction Many people in Pakistan depend on agriculture but various crops are contaminated by phytopathogenic fungi (i.e., Aspergillus, Fusarium, Penicillium) during pre and post-harvesting processes. -

Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest
Asian Journal of Biology 9(2): 19-32, 2020; Article no.AJOB.55647 ISSN: 2456-7124 Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest D. R. B. Sonchita1, F. M. Aminuzzaman1*, A. A. Joty1, J. F. Tanni1, M. N. Islam1 and M. Rahaman1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration among all authors. Author DRBS collected the samples and conducted the research work. Author FMA designed and supervised the research work, collected the samples, wrote and edited the manuscript. Authors AAJ and MNI collected the samples. Authors JFT and MR managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJOB/2020/v9i230084 Editor(s): (1) Dr. P. Dhasarathan, Anna University, India. Reviewers: (1) Blagoy Uzunov, Sofia University “St. Kliment Ohridski”, Bulgaria. (2) Siddhant, Durgesh Nandini Degree College, India. (3) Shengrong Liu, Ningde Normal University, China. Complete Peer review History: http://www.sdiarticle4.com/review-history/55647 Received 20 March 2020 Original Research Article Accepted 27 May 2020 Published 05 June 2020 ABSTRACT Survey on macro fungi was made in Gajni forest, Sherpur, Bangladesh which is located in between 24°18' and 25°18' north latitudes and in between 89°53' and 90°91' east longitudes. It is bounded by Meghalaya state of India on the north, Mymensingh and Jamalpur districts on the south with a wide range of ecosystem. The survey was conducted on July to December, 2018 to identify and preserve wood-rot causal macro fungi for future industrial utilization. -

Department of Plant Pathology Dhaka-1207 June
OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH ARIFA AFRIN JOTY DEPARTMENT OF PLANT PATHOLOGY SHER-E-BANGLAAGRICULTURALUNIVERSITY DHAKA-1207 JUNE, 2018 OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH BY ARIFA AFRIN JOTY REGISTRATION NO. 12-05051 A Thesis Submitted to the Faculty of Agriculture Sher-e-Bangla Agricultural University, Dhaka-1207, in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE (M.S.) IN PLANT PATHOLOGY SEMESTER: JANUARY- JUNE, 2018 Approved by: Dr. F. M. Aminuzzaman Dr. Nazneen Sultana Professor Professor Department of Plant Pathology Department of Plant Pathology Sher-e-Bangla Agricultural University Sher-e-Bangla Agricultural University Supervisor Co- Supervisor Khadija Akhter Chairman Examination Committee ACKNOWLEDGEMENTS All admiration and praises are solely to “Almighty Allah” Whose mercy absolutely enabled the author to pursue the higher study in Agriculture discipline and complete M.S. Course and research work successfully for the degree of M.S. in Plant Pathology. The author expresses her immense respect and deepest sense of gratitude and heartfelt thanks to her most reverend teacher and Supervisor, Professor Dr. F. M. Aminuzzaman, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his untiring and efficient guidance, timely instruction, valuable advices and encouragement throughout the research work and completion of this thesis. The author is grateful to her research Co-Supervisor, Prof. Dr. Nazneen Sultana, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his guidance, keen interest, valuable advices and continuous encouragement regarding this research. The author also wishes to pay her deep respect to Prof. -

Checklist of the Species of the Genera Amanita and Limacella (Agaricomycetes) in Estonia
Folia Cryptog. Estonica, Fasc. 45: 81–85 (2009) Checklist of the species of the genera Amanita and Limacella (Agaricomycetes) in Estonia Mall Vaasma Institute of Agricultural and Environmental Sciences, Estonian University of Life Sciences, 181 Riia St., 51014, Tartu, Estonia. Natural History Museum, University of Tartu, 46 Vanemuise St., 51014, Tartu, Estonia. E-mail: [email protected] Abstract: 19 species, 2 varieties and 1 form of genus Amanita and 3 species of genus Limacella (Agaricomycetes) have been recorded in Estonia. A checklist of these species with habitat, phenology and occurrence data are presented. Kokkuvõte: Kärbseseene (Amanita) ja limalooriku (Limacella) perekonna (Agaricomycetes) liikide kriitiline nimestik Eestis Eestis on kärbseseene perekonnas 19 liiki, 2 teisendit ja 1 vorm, limalooriku perekonnas on 3 liiki. Igale liigile on antud andmed tema kasvukoha, fenoloogia ja esinemissageduse kohta. The present checklist contains 19 Amanita spe- FE – Neville, Poumarat, Fungi Europaei, 2004 cies, 2 varieties and 1 form and 3 Limacella spe- Galli – Galli, 2001 cies recorded in Estonia. All the species included GBW – Krieglsteiner, 2003 have been proved by relevant exsiccata in the KL – Kalamees & Liiv, 2005 mycological herbarium TAAM of the Institute of Korh – Korhonen, 2007 Agricultural and Environmental Sciences of the Lud – Ludwig, 2000 Estonian University of Life Sciences and in the Phil – Pillips, 2006 mycological herbarium TU of the Natural History RH – Ryman & Holmåsen, 2006 Museum of the University of Tartu. According to RM – Rivista di Micologia, 2008 literary sources (Urbonas a.o. 1986) Limacella SNS – Salo, Niemelä & Salo, 2006 delicata (Fr.) Earle has also been recorded in Estonia, but the exsiccata available do not en- AMANITA Pers., Tent. -
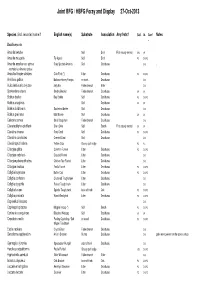
Joint BFG / HBFG Foray and Display 27-Oct-2013
Joint BFG / HBFG Foray and Display 27-Oct-2013 Species (incl. recorded name if English name(s) Substrate Association Any firsts? Coll. Id. Conf Notes . Basidiomycota Amanita betulae Soil Birch First county record SH3 SK Amanita muscaria Fly Agaric Soil Birch PC DJSPC Amanita excelsa var. spissa Grey Spotted Amanita Soil Deciduous DJS . recorded as Amanita spissa Ampulloclitocybe clavipes Club Foot (*) Litter Deciduous PC DJSPC . Armillaria gallica Bulbous Honey Fungus on roots Deciduous DJS Auricularia auricula-judae Jelly Ear Fallen branch Elder DJS Bjerkandera adusta Smoky Bracket Fallen branch Deciduous SK SK Boletus badius Bay Bolete Soil Deciduous PC DJSPC Boletus cisalpinus Soil Deciduous SK SK Boletus luridiformis Scarletina Bolete Soil Deciduous DJS Boletus pruinatus Matt Bolete Soil Deciduous SK SK Calocera cornea Small Stagshorn Fallen branch Deciduous DJS Clavariadelphus pistillaris Giant Club Soil Beech First county record SK SK Clavulina cinerea Grey Coral Soil Deciduous PC DJSPC Clavulina coralloides Crested Coral Soil Deciduous DJS Clavulinopsis helvola Yellow Club Grassy path edge PC PC Clitocybe gibba Common Funnel Litter Deciduous PC DJSPC Clitocybe nebularis Clouded Funnel Litter Deciduous DJS Clitocybe phaeophthalma Chicken Run Funnel Litter Deciduous DJS Clitocybe rivulosa Fool's Funnel Litter Deciduous PC DJSPC Collybia butyracea Butter Cap Litter Deciduous PC DJSPC Collybia confluens Clustered Toughshank Litter Deciduous DJS Collybia dryophila Russet Toughshank Litter Deciduous DJS Collybia fusipes Spindle Toughshank -

Phd. Thesis Sana Jabeen.Pdf
ECTOMYCORRHIZAL FUNGAL COMMUNITIES ASSOCIATED WITH HIMALAYAN CEDAR FROM PAKISTAN A dissertation submitted to the University of the Punjab in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BOTANY by SANA JABEEN DEPARTMENT OF BOTANY UNIVERSITY OF THE PUNJAB LAHORE, PAKISTAN JUNE 2016 TABLE OF CONTENTS CONTENTS PAGE NO. Summary i Dedication iii Acknowledgements iv CHAPTER 1 Introduction 1 CHAPTER 2 Literature review 5 Aims and objectives 11 CHAPTER 3 Materials and methods 12 3.1. Sampling site description 12 3.2. Sampling strategy 14 3.3. Sampling of sporocarps 14 3.4. Sampling and preservation of fruit bodies 14 3.5. Morphological studies of fruit bodies 14 3.6. Sampling of morphotypes 15 3.7. Soil sampling and analysis 15 3.8. Cleaning, morphotyping and storage of ectomycorrhizae 15 3.9. Morphological studies of ectomycorrhizae 16 3.10. Molecular studies 16 3.10.1. DNA extraction 16 3.10.2. Polymerase chain reaction (PCR) 17 3.10.3. Sequence assembly and data mining 18 3.10.4. Multiple alignments and phylogenetic analysis 18 3.11. Climatic data collection 19 3.12. Statistical analysis 19 CHAPTER 4 Results 22 4.1. Characterization of above ground ectomycorrhizal fungi 22 4.2. Identification of ectomycorrhizal host 184 4.3. Characterization of non ectomycorrhizal fruit bodies 186 4.4. Characterization of saprobic fungi found from fruit bodies 188 4.5. Characterization of below ground ectomycorrhizal fungi 189 4.6. Characterization of below ground non ectomycorrhizal fungi 193 4.7. Identification of host taxa from ectomycorrhizal morphotypes 195 4.8. -

Tarset and Greystead Biological Records
Tarset and Greystead Biological Records published by the Tarset Archive Group 2015 Foreword Tarset Archive Group is delighted to be able to present this consolidation of biological records held, for easy reference by anyone interested in our part of Northumberland. It is a parallel publication to the Archaeological and Historical Sites Atlas we first published in 2006, and the more recent Gazeteer which both augments the Atlas and catalogues each site in greater detail. Both sets of data are also being mapped onto GIS. We would like to thank everyone who has helped with and supported this project - in particular Neville Geddes, Planning and Environment manager, North England Forestry Commission, for his invaluable advice and generous guidance with the GIS mapping, as well as for giving us information about the archaeological sites in the forested areas for our Atlas revisions; Northumberland National Park and Tarset 2050 CIC for their all-important funding support, and of course Bill Burlton, who after years of sharing his expertise on our wildflower and tree projects and validating our work, agreed to take this commission and pull everything together, obtaining the use of ERIC’s data from which to select the records relevant to Tarset and Greystead. Even as we write we are aware that new records are being collected and sites confirmed, and that it is in the nature of these publications that they are out of date by the time you read them. But there is also value in taking snapshots of what is known at a particular point in time, without which we have no way of measuring change or recognising the hugely rich biodiversity of where we are fortunate enough to live. -

Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka
Journal of Advances in Biology & Biotechnology 11(4): 1-18, 2017; Article no.JABB.30970 ISSN: 2394-1081 SCIENCEDOMAIN international www.sciencedomain.org Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka H. Rubina1, F. M. Aminuzzaman1*, M. S. M. Chowdhury1 and K. Das1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration between all authors. Author HR wrote the protocol, carried out research and wrote the first draft of the manuscript. Author KD carried out microscopic work and spore morphology. Author FMA designed and supervised the research, identified the fungal genera and also edited the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/JABB/2017/30970 Editor(s): (1) Mohammad Arif, Department of Plant Pathology, Kansas State University, Manhattan, Kansas, USA. Reviewers: (1) Chitta Ranjan Deb, Nagaland University, Lumami, India. (2) Rajesh Kumar, Rain Forest Research Institute, Assam, India. Complete Peer review History: http://www.sciencedomain.org/review-history/17933 Received 12th December 2016 th Original Research Article Accepted 6 February 2017 Published 23rd February 2017 ABSTRACT This investigation was conducted in National Botanical Garden, Dhaka located at 24°00′ N (Latitude), 90°00′ E (Longitude) to document the morphology, diversity and distribution of macro fungi during the rainy seasons of July to October, 2015. A total of 23 macro fungi samples were collected and identified to 20 species under 10 genera and 10 families. The predominant genera were Ganoderma sp., Lepiota sp., Daedeleopsis sp., Russula sp., Psythyrella sp., Lycoperdon sp., Crepidotus sp., Psilocybe sp, Flammulina sp. -

Biodiversity and Morphological Characterization of Mushrooms at the Tropical Moist Deciduous Forest Region of Bangladesh
American Journal of Experimental Agriculture 8(4): 235-252, 2015, Article no.AJEA.2015.167 ISSN: 2231-0606 SCIENCEDOMAIN international www.sciencedomain.org Biodiversity and Morphological Characterization of Mushrooms at the Tropical Moist Deciduous Forest Region of Bangladesh M. I. Rumainul1, F. M. Aminuzzaman1* and M. S. M. Chowdhury1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration with all authors. Author MIR carried out the research and wrote the first draft of the manuscript. Author FMA designed use supervised and edited the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJEA/2015/17301 Editor(s): (1) Sławomir Borek, Department of Plant Physiology, Adam Mickiewicz University, Poland. Reviewers: (1) Anonymous, Ghana. (2) Anonymous, India. (3) Eduardo Bernardi, Departamento de Microbiologia, Universidade Federal de Pelotas, Brazil. Complete Peer review History: http://www.sciencedomain.org/review-history.php?iid=1078&id=2&aid=9182 Received 7th March 2015 th Original Research Article Accepted 17 April 2015 Published 8th May 2015 ABSTRACT Mushroom flora is an important component of the ecosystem and their biodiversity study has been largely neglected and not documented for the tropical moist deciduous forest regions of Bangladesh. This investigation was conducted in seven different areas of tropical moist deciduous forest region of Bangladesh namely Dhaka, Gazipur, Bogra, Rajshahi, Pabna, Jaipurhat and Dinajpur. Mushroom flora associated with these forest regions were collected, photographed and preserved. A total of fifty samples were collected and identified to fourteen genera and twenty four species. -

Checklist of Macrofungal Species from the Phylum
UDK: 582.284.063.7(497.7) Acta Musei Macedonici Scientiarum Naturalium, 2018, Vol. 21, pp: 23-112 Received: 10.07.2018 ISSN: 0583-4988 (printed version) Accepted: 07.11.2018 ISSN: 2545-4587 (on-line version) Review paper Available on-line at: www.acta.musmacscinat.mk Checklist of macrofungal species from the phylum Basidiomycota of the Republic of Macedonia Mitko Karadelev1*, Katerina Rusevska1, Gerhard Kost2, Danijela Mitic Kopanja1 1Institute of Biology, Faculty of Natural Science and Mathematics, Ss Cyril and Methodius University, Skopje, Macedonia 2Department of Systematic Botany and Mycology, Faculty of Biology, Philipps University of Marburg, Germany *corresponding author’s e-mail: [email protected] Abstract The interest in macrofungal studies in Macedonia has been growing in the past 20 years. The data sources used are published data, exsiccatae and notes from our own studies, as well as specimens from other collectors. According to the research conducted up to now, a total of 1,735 species, 27 varieties and 4 forms of Basidiomycota have been recorded in the country. A large part of this data is a result of the field and taxonomic work in the last two decades. This paper includes 497 taxa new to Macedonia. Key words: fungi, Macedonian macrofungi diversity, nomenclature, taxonomy. Introduction array of regions in Macedonia, such as Pelister, Jakupi- ca, Galichica, Golem Grad Island, Kozuf, Shar Planina From a mycological perspective, the Republic of and South Povardarie, mainly lignicolous species of Macedonia has been studied reasonably well. A num- fungi were studied (Tortić 1988; Karadelev 1993, ber of publications have been made by foreign mycolo- 1995c, d; Karadelev, Rusevska 2000; Karadelev et al. -

The Diversity of Fungi in Four Irish Forest Types by Richard O'hanlon B.Sc
The diversity of fungi in four Irish forest types By Richard O’Hanlon B.Sc. (Ed) A thesis submitted for the degree of Doctor of Philosophy, At the Faculty of Science and Engineering, University of Limerick, Ireland. Supervisor: Dr Thomas Harrington, Department of Life Sciences, University of Limerick. Submitted to the University of Limerick: May 2011 i ii “The task of an ecologist” There is an old story about a man who, returning home one night found his neighbour searching the ground beneath a street lamp. “Can I help you find something?” he asked. “I lost my key” replied the neighbour. “Do you know about where you dropped it?”, “Yes” replied the neighbour “over there” pointing to a dark corner of the street. “If you dropped it over there then why are you looking here” asked the man. “Because this is where the light is” replied the neighbour. The task of the ecologist is not to bring the search to where the light is, but to bring the light to where the search is. Perry et al. (2008) iii iv Abstract Sampling of the macrofungal sporocarps, ectomycorrhizal morphotypes and vascular plants was carried out in 28 plots from four forest types (ash, oak, Scot’s pine, Sitka spruce) between the years 2007 and 2009. A total of 409 macrofungal species, 51 ectomycorrhizal morphotypes and 68 vascular plant species were recorded over the three years. It was found that at equal sampling intensities, there were no significant differences in total macrofungal species or ectomycorrhizal morphotype richness between the oak, Scot’s pine and Sitka spruce forest types.