Annual Report 2019-20 CCRAS ANNUAL REPORT 2019-20 CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommendations on Improving Telecom Services in Andaman
Telecom Regulatory Authority of India Recommendations on Improving Telecom Services in Andaman & Nicobar Islands and Lakshadweep 22 nd July, 2014 Mahanagar Doorsanchar Bhawan Jawahar Lal Nehru Marg, New Delhi – 110002 CONTENTS CHAPTER-I: INTRODUCTION 1 CHAPTER- II: METHODOLOGY FOLLOWED FOR THE ASSESSMENT OF THE TELECOM INFRASTRUCTURE REQUIRED 10 CHAPTER- III: TELECOM PLAN FOR ANDAMAN & NICOBAR ISLANDS 36 CHAPTER- IV: COMPREHENSIVE TELECOM PLAN FOR LAKSHADWEEP 60 CHAPTER- V: SUPPORTING POLICY INITIATIVES 74 CHAPTER- VI: SUMMARY OF RECOMMENDATIONS 84 ANNEXURE 1.1 88 ANNEXURE 1.2 90 ANNEXURE 2.1 95 ANNEXURE 2.2 98 ANNEXURE 3.1 100 ANNEXURE 3.2 101 ANNEXURE 5.1 106 ANNEXURE 5.2 110 ANNEXURE 5.3 113 ABBREVIATIONS USED 115 i CHAPTER-I: INTRODUCTION Reference from Department of Telecommunication 1.1. Over the last decade, the growth of telecom infrastructure has become closely linked with the economic development of a country, especially the development of rural and remote areas. The challenge for developing countries is to ensure that telecommunication services, and the resulting benefits of economic, social and cultural development which these services promote, are extended effectively and efficiently throughout the rural and remote areas - those areas which in the past have often been disadvantaged, with few or no telecommunication services. 1.2. The Role of telecommunication connectivity is vital for delivery of e- Governance services at the doorstep of citizens, promotion of tourism in an area, educational development in terms of tele-education, in health care in terms of telemedicine facilities. In respect of safety and security too telecommunication connectivity plays a vital role. -

Tla Hearing Board
TLA HEARING BOARD Hearing Schedule from 01/10/2019 to 31/10/2019 Location: DELHI Hearing Timing : 10.30 am to 1.00 pm S.No TM No Class Hearing Proprietor Name Agent Name Mode of Date Hearing 1 3408747 41 01-10-2019 GAURAV SHARGA KSHITIJ MALHOTRA Physical 2 2713186 25 01-10-2019 TV TODAY NETWORK LTD. SAJAD SULTAN ADV., Physical 3 3404419 1 01-10-2019 TV TODAY NETWORK LIMITED SAJAD SULTAN ADV., Physical 4 3404420 2 01-10-2019 TV TODAY NETWORK LIMITED SAJAD SULTAN ADV., Physical 5 3425744 5 01-10-2019 MR. ASHISH KUMAR DUBEY LALJI ADVOCATES Physical 6 3426307 7 01-10-2019 SH. SHYAM VERMA. LALJI ADVOCATES Physical 7 3426308 11 01-10-2019 SH. SHYAM VERMA. LALJI ADVOCATES Physical 8 3426309 30 01-10-2019 PREM SINGH. LALJI ADVOCATES Physical 9 3426310 43 01-10-2019 PREM SINGH. LALJI ADVOCATES Physical 10 3426312 12 01-10-2019 SH. PAWAN KUMAR GUPTA. LALJI ADVOCATES Physical 11 3426314 12 01-10-2019 KSHITIZ GUPTA. LALJI ADVOCATES Physical 12 3427343 12 01-10-2019 MANINDER SINGH. LALJI ADVOCATES Physical 13 3427349 42 01-10-2019 MOBIN SIGNITY SOLUTIONS PRIVATE LIMITED. LALJI ADVOCATES Physical 14 3427353 6 01-10-2019 PANKAJ MITTAL. LALJI ADVOCATES Physical 15 3427356 35 01-10-2019 UNIQUE LIFE SCIENCES PVT. LTD. LALJI ADVOCATES Physical 16 3429261 9 01-10-2019 SAURABH GROVER. LALJI ADVOCATES Physical 17 3429262 7 01-10-2019 SH. RAM JAGAT. LALJI ADVOCATES Physical 18 3429263 11 01-10-2019 SH. RAM JAGAT. LALJI ADVOCATES Physical 19 3405602 5 01-10-2019 ALEXA PHARMACEUTICALS PRIVATE LIMITED ALEXA Physical PHARMACEUTICALS PRIVATE LIMITED 20 3417160 6 01-10-2019 SH. -

November 17-2
Tuesday 2 Daily Telegrams November 17, 2020 GOVT. PRIMARY SCHOOL No. TN/DB/PHED/2020/1277 27 SUBHASGRAM - 2 HALDER PARA, SARDAR TIKREY DO OFFICE OF THE EXECUTIVE ENGINEER NSV, SUBHASHGRAM GOVT. PRIMARY SCHOOL PUBLIC HEALTH ENGINEERING DIVISION 28 SUBHASGRAM - 3 DAS PARA, DAKHAIYA PARA DO A.P.W.D., PORT BLAIR NSV, SUBHASHGRAM th SCHOOL TIKREY, SUB CENTER Prothrapur, dated the 13 November 2020. COMMUNITY HALL, 29 KHUDIRAMPUR AREA, STEEL BRIDGE, AAGA DO KHUDIRAMPUR TENDER NOTICE NALLAH, DAM AREA (F) The Executive Engineer, PHED, APWD, Prothrapur invites on behalf of President of India, online Item Rate e- BANGLADESH QUARTER, MEDICAL RAMAKRISHNAG GOVT. PRIMARY SCHOOL tenders (in form of CPWD-8) from the vehicle owners / approved and eligible contractors of APWD and Non APWD 30 COLONY AREA, SAJJAL PARA, R K DO RAM - 1 RAMKRISHNAGRAM Contractors irrespective of their enlistment subject to the condition that they have experience of having successfully GRAM HOUSE SITE completed similar nature of work in terms of cost in any of the government department in A&N Islands and they should GOVT. PRIMARY SCHOOL RAMAKRISHNAG BAIRAGI PARA, MALO PARA, 31 VV PITH, DO not have any adverse remarks for following work RAM - 2 PAHAR KANDA NIT No. Earnest RAMKRISHNAGRAM Sl. Estimated cost Time of Name of work Money RAMAKRISHNAG COMMUNITY HALL, NEAR MAGAR NALLAH WATER TANK No. put to Tender Completion 32 DO Deposit RAM - 3 VKV, RAMKRISHNAGRAM AREA, POLICE TIKREY, DAS PARA VIDYASAGARPAL GOVT. PRIMARY SCHOOL SAITAN TIKRI, PANDEY BAZAAR, 1 NIT NO- R&M of different water pump sets under 33 DO 15/DB/ PHED/ E & M Sub Division attached with EE LI VS PALLY HELIPAD AREA GOVT. -
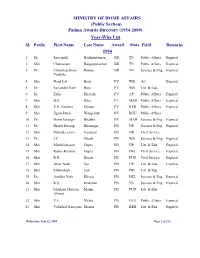
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

6. Secretary, Ministry of Minority Affairs, 11Trr Floor, Paryavaran Bhawan, CGO Complex, Lodhi Road, New Delhi -110003 7
F.No.14-2/2O2O-[S.L Government of India Ministry of Human Resource Development Department of School Education& Literacy Shastri Bhawan, New Delhi Date;1)lluily,2ozo subject: Minutes ofthe meeting ofthe proiect Approval Board held on 17s March, 2O2O to consider the Annual Work plan & Budget (AWP&B) ZO2O-ZL ol Samagra Shiksha for the UT ofAndaman & Nicobar Islands-reg. The Meeting of Project Approval Board (pAB) of samagra shiksha was held on L7 .03.2020 for under the chairmanship of secretary (sE&L) in New Delhi to consider the Annual work Plan & Budget (Awp&B), zo2o-zt for the ur of Andaman & Nicobar Islands. 2. The undersigned is directed to forward herewith the approved copy of pAB Minutes in respect of samagra shiksha, ur of Andaman & Nicobar Istands for the year 2020-2L for further necessary action. Encl: As above (H. Sonkusare) Under Secretary to the Government of India Phr Ott-25387342 To, 1. Secretary, Ministry of W&CD, 2. Secretary, Ministry of Labour & Employment. 3. Secretary, Ministry of Social fustice & Empowerment. 4. Secretary, Ministry of Tribal Affairs. 5. Secretary, Ministry of Drinking Water & Sanitation, 4th floor, paryavaran Bhawan, CGO compleS Lodhi Road, New Dethi -110O03 6. Secretary, Ministry of Minority Affairs, 11trr floor, paryavaran Bhawan, CGO complex, Lodhi Road, New Delhi -110003 7. secretary, Department of Empowerment of persons with Disabilities, Ministry of Social fustice & Empowerment 8. Deputy Adviser (Education), NITI Aayog 9. Director, NCERT 10. Vice Chancello4 NIEPA Page | 1 11. Chairperson, NCTE, Hans Bhawan, Wing II, 1 Bahadur Shah Zafar Marg, New Delhi -110002 LZ.Yice Chancellor,IGNOU, Maidan Garhi, New Delhi 13. -
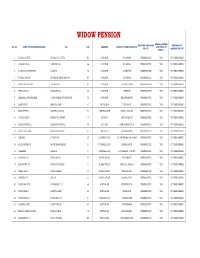
WIDOW COMBINED LIST.Xlsx
WIDOW PENSION ANNUAL INCOME WHETHER EMPLOYED WHETHER RE- SL. NO. NAME OF THE BENEFICIARIES W/o AGE ADDRESS NAME OF GRAM PANCHAYAT LESS THAN RS OR NOT MARRIED OR NOT 48000 /- 1 SUBALA DUTTA GOPAL CH. DUTTA 62 DIGLIPUR R K GRAM UNEMPLOYED YES NOT REMARRIED 2 SUBARNA DAS DHIREN DAS 86 DIGLIPUR R K GRAM UNEMPLOYED YES NOT REMARRIED 3 SUCHILA RAJBANGSHI LAXMAN 76 DIGLIPUR LAXMIPUR UNEMPLOYED YES NOT REMARRIED 4 SUNILA BISWAS SUDHIR KUMAR BISWAS 83 DIGLIPUR R K GRAM UNEMPLOYED YES NOT REMARRIED 5 SWARNA LATA ROY JADAB ROY 81 DIGLIPUR SUBASH GRAM UNEMPLOYED YES NOT REMARRIED 6 SEFALI BALA SANKAR BALA 40 DIGLIPUR SHIBPUR UNEMPLOYED YES NOT REMARRIED 7 AMILIBALA SWARNAKAR AJITH KUMAR SWARNAKAR 50 DIGLIPUR KHUDIRAMPUR UNEMPLOYED YES NOT REMARRIED 8 LALITA GAIN MANGAL GAIN 51 SHOAL BAY SHOAL BAY UNEMPLOYED YES NOT REMARRIED 9 MARRY TETE MARSHAL KULLU 72 SUNDERGARH KHARA NALLAH UNEMPLOYED YES NOT REMARRIED 10 JASINTA EKKA NIRBRITUS TIRKEY 57 HUTBAY CEYLON BASTI UNEMPLOYED YES NOT REMARRIED 11 SABITA MONDAL PRAKASH MONDAL 55 HUT BAY RAM KRISHNAPUR UNEMPLOYED YES NOT REMARRIED 12 ARATI BALA DAS KUNJESWAR DAS 51 DIGLIPUR KHUDIRAMPUR UNEMPLOYED YES NOT REMARRIED 13 LAKSHMI OYYAPPAN 45 CAMPBELL BAY JOGINDER NAGAR C/BAY UNEMPLOYED YES NOT REMARRIED 14 RAJESHWARI. M MUTHURAMALINGA 51 CAMPBELL BAY KAMAL BASTI UNEMPLOYED YES NOT REMARRIED 15 YELAMMA BAIRAGI 57 CAMPBELL BAY FISHERMAN COLONY UNEMPLOYED YES NOT REMARRIED 16 ACHAMMA. B SIMADARI. B 77 BAMBOOFLAT NAYA BASTI UNEMPLOYED YES NOT REMARRIED 17 EMAWATHY. M VENKATA SWAMY. 51 BAMBOOFLAT MEDICAL PAHAD UNEMPLOYED YES NOT REMARRIED 18 TAMIL SELVI VIJAY KUMAR 43 BAMBOOFLAT WRIGHT MYO UNEMPLOYED YES NOT REMARRIED 19 SAKEENA. -
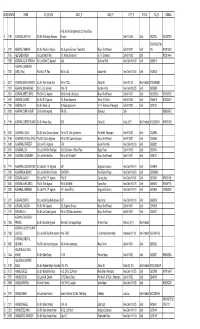
Main Voter List 08.01.2018.Pdf
Sl.NO ADM.NO NAME SO_DO_WO ADD1_R ADD2_R CITY_R STATE TEL_R MOBILE 61-B, Abul Fazal Apartments 22, Vasundhara 1 1150 ACHARJEE,AMITAVA S/o Shri Sudhamay Acharjee Enclave Delhi-110 096 Delhi 22620723 9312282751 22752142,22794 2 0181 ADHYARU,YASHANK S/o Shri Pravin K. Adhyaru 295, Supreme Enclave, Tower No.3, Mayur Vihar Phase-I Delhi-110 091 Delhi 745 9810813583 3 0155 AELTEMESH REIN S/o Late Shri M. Rein 107, Natraj Apartments 67, I.P. Extension Delhi-110 092 Delhi 9810214464 4 1298 AGARWAL,ALOK KRISHNA S/o Late Shri K.C. Agarwal A-56, Gulmohar Park New Delhi-110 049 Delhi 26851313 AGARWAL,DARSHANA 5 1337 (MRS.) (Faizi) W/o Shri O.P. Faizi Flat No. 258, Kailash Hills New Delhi-110 065 Delhi 51621300 6 0317 AGARWAL,MAM CHANDRA S/o Shri Ram Sharan Das Flat No.1133, Sector-29, Noida-201 301 Uttar Pradesh 0120-2453952 7 1427 AGARWAL,MOHAN BABU S/o Dr. C.B. Agarwal H.No. 78, Sukhdev Vihar New Delhi-110 025 Delhi 26919586 8 1021 AGARWAL,NEETA (MRS.) W/o Shri K.C. Agarwal B-608, Anand Lok Society Mayur Vihar Phase-I Delhi-110 091 Delhi 9312059240 9810139122 9 0687 AGARWAL,RAJEEV S/o Shri R.C. Agarwal 244, Bharat Apartment Sector-13, Rohini Delhi-110 085 Delhi 27554674 9810028877 11 1400 AGARWAL,S.K. S/o Shri Kishan Lal 78, Kirpal Apartments 44, I.P. Extension, Patparganj Delhi-110 092 Delhi 22721132 12 0933 AGARWAL,SUNIL KUMAR S/o Murlidhar Agarwal WB-106, Shakarpur, Delhi 9868036752 13 1199 AGARWAL,SURESH KUMAR S/o Shri Narain Dass B-28, Sector-53 Noida, (UP) Uttar Pradesh0120-2583477 9818791243 15 0242 AGGARWAL,ARUN S/o Shri Uma Shankar Agarwal Flat No.26, Trilok Apartments Plot No.85, Patparganj Delhi-110 092 Delhi 22433988 16 0194 AGGARWAL,MRIDUL (MRS.) W/o Shri Rajesh Aggarwal Flat No.214, Supreme Enclave Mayur Vihar Phase-I, Delhi-110 091 Delhi 22795565 17 0484 AGGARWAL,PRADEEP S/o Late R.P. -

Lucknow Zone CSC List.Xlsx
Lucknow Zone CSC List Sl. Grampanchayat District Block Name Village/CSC name Pincode Location VLE Name Contact No No. Village Name 1 Sultanpur Sultanpur4 JAISINGHPUR(R) 228125 ISHAQPUR DINESH ISHAQPUR 730906408 2 Sultanpur Baldirai Bhawanighar 227815 Bhawanighar Sarvesh Kumar Yadav 896097886 3 Hardoi HARDOI1 Madhoganj 241301 Madhoganj Bilgram Road Devendra Singh Jujuvamau 912559307 4 Balrampur Balrampur BALRAMPUR(U) 271201 DEVI DAYAL TIRAHA HIMANSHU MISHRA TERHI BAZAR 912594555 5 Sitapur Sitapur Hargaon 261121 Hargaon ashok kumar singh Mumtazpur 919283496 6 Ambedkar Nagar Bhiti Naghara 224141 Naghara Gunjan Pandey Balal Paikauli 979214477 7 Gonda Nawabganj Nawabganj gird 271303 Nawabganj gird Mahmood ahmad 983850691 8 Shravasti Shravasti Jamunaha 271803 MaharooMurtiha Nafees Ahmad MaharooMurtiha 991941625 9 Badaun Budaun2 Kisrua 243601 Village KISRUA Shailendra Singh 5835005612 10 Badaun Gunnor Babrala 243751 Babrala Ajit Singh Yadav Babrala 5836237097 11 Bareilly Bareilly2 Bareilly Npp(U) 243201 TALPURA BAHERI JASVEER GIR Talpura 7037003700 12 Bareilly Bareilly3 Kyara(R) 243001 Kareilly BRIJESH KUMAR Kareilly 7037081113 13 Bareilly Bareilly5 Bareilly Nn 243003 CHIPI TOLA MAHFUZ AHMAD Chipi tola 7037260356 14 Bareilly Bareilly1 Bareilly Nn(U) 243006 DURGA NAGAR VINAY KUMAR GUPTA Nawada jogiyan 7037769541 15 Badaun Budaun1 shahavajpur 243638 shahavajpur Jay Kishan shahavajpur 7037970292 16 Faizabad Faizabad5 Askaranpur 224204 Askaranpur Kanchan ASKARANPUR 7052115061 17 Faizabad Faizabad2 Mosodha(R) 224201 Madhavpur Deepchand Gupta Madhavpur -

Scanned Image
area war fala year ANDAMAN AND NICOBAR ADMINISTRATION fear Freee /Directorate Of Education kkk 24© lu Port Blair, dated the December, 2020 ORDER No.:- 2OQA i The following transfers and postings of the Primary School Teacher (Hindi/English Medium) are hereby ordered as under: S.No. Name ofthe Staff From | To Remarks Ms.Salma PS Aerial [11-01-1988]Bibi Bay MS Brichgunj Mr.Abdul Majeed K _ PS Shantanu PS Badmaspahar Ms.Amina Bibi.C | PS Shantanu [02-11-1978] SSS Model Port Blair _ | Ms.Archana Devi SS Nimbutala PS Aerial Bay Ms.Maimuna.K PS S.V. Mandir SS Tamaloo Mr.Sadakat Ali MS Kalsi PS Break Water Ms.Sandhya Singh PS Sabari Jn. MS Humfrygunj Ms. Anjali Devi.V PS Sabari Jn. SSS Girls | Ms.Magdeline Boniface SS Harminder Bay MS Kinmai PS Ms.Nahore PS Japan Tikri Headquarter (Model) (Car Nicobar) PS Swaraj Dweep 1/2 11 Ms.Beena Pandey PS (K/Nagar) 6 Mannarghat 12 Ms.Bhagam | Priya SS Sivapuram SSS Long Island [On Request] 13 Mr.Venu Gopal.B SS Kaushalya Nagar PS Lal Pahar 14 Ms.Priya [12-04-1981] SS Kaushalya Nagar MS Humfrygunj Ms.Aruna.G 15 PS Aerial Bay PS Anna Nagar | 16 Ms.Jyothi.S.P PS Aerial Bay SSS Garacharma “Mr 17 Rajoni Kanto Ojha SS Ram Nagar-| PS Shantanu Ms.Rasheena Bibi 18 SS Ram Nagar-| SSS Hutbay (Model) 19 Mr.Mohd. Amzad Khan SS Ram Nagar-| PS Narayan Tikri Ms.Manju Kumari 20 PS Subhash Gram PS [28-10-1976] Attampahar 21 Mr.Wilson PS Pillowolo MS Minyuk SSS Swaraj Dweep 22 Ms.Papiya Das PS Subhash (Havelock) Gram 23 Ms.Shahnaz Bibi.K.P PS Rajat Garh PS Aerial Bay | Ms. -

Indigenous Treatment of Pancreatitis
Amar was formulated by Late Vaidya Chandra Prakash ji from Meerut using Copper, Mercury and Sulphur in the seventies. Incidentally, the formulation brought miraculous recovery in an advanced stage patient of pancreatic cancer. Late Vaidya Chandra Prakash ji Later, Vaidya Balendu Prakash (his son) revived the formula and observed its miraculous effect in treating certain diseases. He noted that the formulation brought complete and sustainable relief to patients of Recurrent Acute/ Chronic Pancreatitis (RACP) and started documenting his clinical practice for these indications following good clinical practice guidelines. This booklet is aimed at disseminating the information collected from the clinical pra tice and scientific developments so far. We are grateful to the following for their contribution towards the making of this booklet: Concept & Editor in Chief: Vaidya Balendu Prakash Patient Consultation & Treatment: Vaidya Balendu Prakash, Vaidya Shikha Prakash Patient Counselling & Data Generation: Ms Shakshi Sharma, Ms Kajol Lal, Ms Neha Negi, Ms Karuna Swarup, Ms Ragini Verma Preparation of Medicines: Mrs Gopa Indu, Mr Devendu Prakash Consultancy: Ms Megha Prakash Data Analysis, Content & Editing: Mrs Sneha Tiwari Sati Design & Layout: Ballabh & Hari Contents Foreword 1 Messages from Dignitaries 3 Editorial 8 The Pancreas and Pancreatic Disorders 11 Pancreatitis – Historical Perspective 12 Pancreatitis 15 Causes of Pancreatitis 16 Conventional Treatment and Prognosis 16 Our Experience with Pancreatitis (data from clinical practice) -

Omnibus Omnibus List
Omnibus List List of All Applicants who have submitted Entrance Application for Admission in the PG/B.Ed. Programmes. PG/B.Ed. काय मों म वेश के िलए वेश आवेदन जमा करने वाले सभी आवेदकों की सूची। Note: 1. Applicants are required to input/edit correct percent of UG Class on or before 21/10/2020. Ignore if already correct percent has been edited. 2. In case of wrong percent or blank percent, application will be rejected. 3. 1st Merit List will be announced on 22/10/2020 in the university website. ान द: 1. आवेदकों को 21/10/2020 तक या उससे पहले UG Class के सही ितशत का इनपुट / संपादन करना आव क है। अगर पहले से ही सही ितशत संपािदत िकया गया है तो अनदेखा कर| 2. गलत ितशत या र ितशत होने पर, आवेदन अ ीकार कर िदया जाएगा। 3. पहली मे रट सूची की घोषणा 22/10/2020 को िव िव ालय की वेबसाइट म की जाएगी। PG/B.Ed. पा म म वेश लेने वाले सभी आवेदक (जो अभी तक UG Class का correct percent इनपुट नह कये है) को सूिचत कया जाता है क UG Class के result का ितशत अितम ितिथ (21/10/2020) के पहले Input/Edit/Update करे | UG Class के percent के आधार पर merit list बनेगी | UG class का correct percent Input/edit नह करने पर Entrance Form अमा य हो जायेगा | Click bellow link to Edit/Update percent http://admission.igntuonline.in/Entrance/App_Login.aspx Click bellow link for How to Edit Form manual http://admission.igntuonline.in/EditForm2020.pdf Application Percent in S.No. -
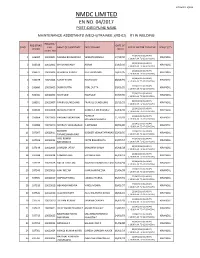
Nmdc Limited En No
PAGE NO.1/102 NMDC LIMITED EN NO. 04/2017 POST /DISCIPLINE NAME MAINTENANCE ASSISTANTS (MECH)(TRAINEE )(RS-02) ITI IN WELDING ROLL NO / REGISTRATI DATE OF SLNO HALL NAME OF CANDIDATE FATHER NAME DATE OF WRITTEN TEST & TIME VENUE CITY ON NO BIRTH TICKET NO 22/04/2018 (SUNDAY) 1 336304 10020001 NARLAGIRI RAJENDAR VENKATESWARLU 17/10/90 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 2 336344 10020002 BHAGVANSINGH ASHOK 23/05/96 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 3 336477 10020003 RISHIKESH KUNDU KARTICK KUNDU 14/01/95 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 4 336478 10020004 SANJIT SHOW RAM SHOW 08/06/95 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 5 336480 10020005 SUBIR DUTTA SITAL DUTTA 30/03/95 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 6 336481 10020006 SUJIT SAW RAM SAW 07/07/90 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 7 336551 10020007 PANKAJ DUNGDUNG PRAFULL DUNGDUNG 28/11/93 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 8 336816 10020008 BONDALA VINAY BONDALA SRI RAMULU 12/08/98 KIRANDUL ( 10.00 A.M. TO 12.00 NOON.) PARMAR 22/04/2018 (SUNDAY) 9 336864 10020009 PARMAR SAGARBHAI 21/11/93 KIRANDUL MAHENDRAKUMAR ( 10.00 A.M. TO 12.00 NOON.) 22/04/2018 (SUNDAY) 10 336984 10020010 BOBBADI SANKARARAO B APPARAO 08/06/86 KIRANDUL ( 10.00 A.M.