Chapter 6. Endocrine System. (Complete)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fosavance, INN-Alendronic Acid and Colecalciferol
EMA/175858/2015 EMEA/H/C/000619 EPAR summary for the public Fosavance alendronic acid and colecalciferol This is a summary of the European public assessment report (EPAR) for Fosavance. It explains how the Committee for Medicinal Products for Human Use (CHMP) assessed the medicine to reach its opinion in favour of granting a marketing authorisation and its recommendations on the conditions of use for Fosavance. What is Fosavance? Fosavance is a medicine that contains two active substances: alendronic acid and colecalciferol (vitamin D3). It is available as tablets (70 mg alendronic acid and 2,800 international units [IU] colecalciferol; 70 mg alendronic acid and 5,600 IU colecalciferol). What is Fosavance used for? Fosavance (containing either 2,800 or 5,600 IU colecalciferol) is used to treat osteoporosis (a disease that makes bones fragile) in women who have been through the menopause and are at risk of low vitamin D levels. Fosavance 70 mg/5,600 IU is for use in patients who are not taking vitamin D supplements. Fosavance reduces the risk of fractures (broken bones) in the spine and the hip. The medicine can only be obtained with a prescription. How is Fosavance used? The recommended dose of Fosavance is one tablet once a week. It is intended for long-term use. The patient must take the tablet with a full glass of water (but not mineral water), at least 30 minutes before any food, drink or other medicines (including antacids, calcium supplements and vitamins). To avoid irritation of the oesophagus (the tube that leads from the mouth to the stomach), the patient should not lie down until after their first food of the day, which should be at least 30 minutes after taking the tablet. -

Adrovance, INN-Alendronic Acid
EMA/194587/2011 EMEA/H/C/000759 EPAR summary for the public Adrovance alendronic acid / colecalciferol This is a summary of the European public assessment report (EPAR) for Adrovance. It explains how the Committee for Medicinal Products for Human Use (CHMP) assessed the medicine to reach its opinion in favour of granting a marketing authorisation and its recommendations on the conditions of use for Adrovance. What is Adrovance? Adrovance is a medicine that contains two active substances: alendronic acid and colecalciferol (vitamin D3). It is available as white tablets (capsule-shaped: 70 mg alendronic acid and 2,800 international units [IU] colecalciferol; rectangular: 70 mg alendronic acid and 5,600 IU colecalciferol). What is Adrovance used for? Adrovance (containing either 2,800 or 5,600 IU colecalciferol) is used to treat osteoporosis (a disease that makes bones fragile) in women who have been through the menopause and are at risk of low vitamin D levels. Adrovance 70 mg/5,600 IU is for use in patients who are not taking vitamin D supplements. Adrovance reduces the risk of broken bones in the spine and the hip. The medicine can only be obtained with a prescription. How is Adrovance used? The recommended dose of Adrovance is one tablet once a week. It is intended for long-term use. The patient must take the tablet with a full glass of water (but not mineral water), at least 30 minutes before any food, drink or other medicines (including antacids, calcium supplements and vitamins). To avoid irritation of the oesophagus (gullet), the patient should not lie down until after their first food of the day, which should be at least 30 minutes after taking the tablet. -

Re-Evaluated Data of Dissociation Constants of Alendronic, Pamidronic and Olpadronic Acids
Cent. Eur. J. Chem. • 7(1) • 2009 • 8-13 DOI: 10.2478/s11532-008-0099-z Central European Journal of Chemistry Re-evaluated data of dissociation constants of alendronic, pamidronic and olpadronic acids Invited Paper Alexander P. Boichenko1, Vadim V. Markov1, Hoan Le Kong1, Anna G. Matveeva2, Lidia P. Loginova1* 1Department of Chemical Metrology, Kharkov V.N. Karazin National University, 61077 Kharkov, Ukraine. 2A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 119991 Moscow, Russian Federation Received 01 April 2008; Accepted 23 October 2008 Abstract: The dissociation constants of (4-amino-1-hydroxybutylidene)bisphosphonic (alendronic) acid, (3-(dimethylamino)-1- hydroxypropylidene)bisphosphonic (olpadronic) acid and (3-amino-1-hydroxypropylidene)bisphosphonic (pamidronic) acid were obtained in aqueous solutions (0.10 М КСl) and micellar solutions of cetylpyridinium chloride (0.10 М CPC) at 25.0°C. With the exception of the third dissociation constant of alendronic acid, the dissociation constants of alendronic, olpadronic and pamidronic acids in aqueous solutions matched literature data. The possibility of sodium alendronate determination by acid-base titration by NaOH solution was theoretically grounded on the basis of re-evaluated data of alendronic acid dissociation constants. Keywords: Alendronate • Olpadronate, pamidronate, dissociation constant • Micellar media effect © Versita Warsaw and Springer-Verlag Berlin Heidelberg. 1. Introduction spectrometric [12], refractive index [13] and Sodium salts of (4-amino-1-hydroxybutylidene) conductometric [14] detection have been proposed. bisphosphonic (alendronic) acid, (3-(dimethylamino)- The anion-exchange chromatography with refractive 1-hydroxypropylidene)bisphosphonic (olpadronic) acid index detection is recommended by British and (3-amino-1-hydroxypropylidene)bisphosphonic Pharmacopoeia for the determination of sodium (pamidronic) acid are successfully used for the medical alendronate [15]. -

6. Endocrine System 6.1 - Drugs Used in Diabetes Also See SIGN 116: Management of Diabetes, 2010
1 6. Endocrine System 6.1 - Drugs used in Diabetes Also see SIGN 116: Management of Diabetes, 2010 http://www.sign.ac.uk/guidelines/fulltext/116 Insulin Prescribing Guidance in Type 2 Diabetes http://www.fifeadtc.scot.nhs.uk/media/6978/insulin-prescribing-in-type-2-diabetes.pdf 6.1.1 Insulins (Type 2 Diabetes) 6.1.1.1 Short Acting Insulins 1st Choice – Insuman® Rapid (Human Insulin) – Humulin S® – Actrapid® 2nd Choice – Insulin Aspart (NovoRapid®) (Insulin Analogues) – Insulin Lispro (Humalog®) 6.1.1.2 Intermediate and Long Acting Insulins 1st Choice – Isophane Insulin (Insuman Basal®) (Human Insulin) – Isophane Insulin (Humulin I®) – Isophane Insulin (Insulatard®) 2nd Choice – Insulin Detemir (Levemir®) (Insulin Analogues) – Insulin Glargine (Lantus®) Biphasic Insulins 1st Choice – Biphasic Isophane (Human Insulin) (Insuman Comb® ‘15’, ‘25’,’50’) – Biphasic Isophane (Humulin M3®) 2nd Choice – Biphasic Aspart (Novomix® 30) (Insulin Analogues) – Biphasic Lispro (Humalog® Mix ‘25’ or ‘50’) Prescribing Points For patients with Type 1 diabetes, insulin will be initiated by a diabetes specialist with continuation of prescribing in primary care. Insulin analogues are the preferred insulins for use in Type 1 diabetes. Cartridge formulations of insulin are preferred to alternative formulations Type 2 patients who are newly prescribed insulin should usually be started on NPH isophane insulin, (e.g. Insuman Basal®, Humulin I®, Insulatard®). Long-acting recombinant human insulin analogues (e.g. Levemir®, Lantus®) offer no significant clinical advantage for most type 2 patients and are much more expensive. In terms of human insulin. The Insuman® range is currently the most cost-effective and preferred in new patients. KEY:- H – Hospital Use Only S – Specialist Initiation or Recommendation R – Restricted Use Only Fife Formulary February 2014 Last Amended June 2015 2 Patients already established on insulin should not be switched to alternative products unless recommended by a diabetes specialist. -

Phvwp Class Review Bisphosphonates and Osteonecrosis of the Jaw (Alendronic Acid, Clodronic Acid, Etidronic Acid, Ibandronic
PhVWP Class Review Bisphosphonates and osteonecrosis of the jaw (alendronic acid, clodronic acid, etidronic acid, ibandronic acid, neridronic acid, pamidronic acid, risedronic acid, tiludronic acid, zoledronic acid), SPC wording agreed by the PhVWP in February 2006 Section 4.4 Pamidronic acid and zoledronic acid: “Osteonecrosis of the jaw has been reported in patients with cancer receiving treatment regimens including bisphosphonates. Many of these patients were also receiving chemotherapy and corticosteroids. The majority of reported cases have been associated with dental procedures such as tooth extraction. Many had signs of local infection including osteomyelitis. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene). While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop osteonecrosis of the jaw while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of osteonecrosis of the jaw. Clinical judgement of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment.” Remaining bisphosphonates: “Osteonecrosis of the jaw, generally associated with tooth extraction and/or local infection (including osteomyelits) has been reported in patients with cancer receiving treatment regimens including primarily intravenously administered bisphophonates. Many of these patients were also receiving chemotherapy and corticosteroids. Osteonecrosis of the jaw has also been reported in patients with osteoporosis receiving oral bisphophonates. A dental examination with appropriate preventive dentistry should be considered prior to treatment with bisphosphonates in patients with concomitant risk factors (e.g. -
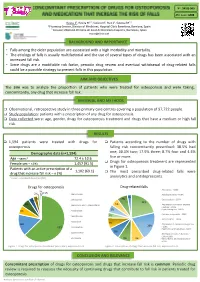
Poster EAHP 140320
Nº: 5PSQ-069 ATC code: M05 Roura J1, Rovira M1,2, Socoro N1, Ruiz S1, Sotoca JM1,2 1 Pharmacy Service, Division of Medicines, Hospital Clínic Barcelona, Barelona, Spain 2 Consorci d’Atenció Primària de Salut de Barcelona Esquerra, Barcelona, Spain [email protected] BACKGROUND AND IMPORTANCE • Falls among the older population are associated with a high morbidity and mortality. • The etiology of falls is usually multifactorial and the use of several types of drugs has been associated with an increased fall risk. • Since drugs are a modifiable risk factor, periodic drug review and eventual withdrawal of drug-related falls could be a possible strategy to prevent falls in this population. AIM AND OBJECTIVES The aim was to analyze the proportion of patients who were treated for osteoporosis and were taking, concomitantly, any drug that increase fall risk. MATERIAL AND METHODS q Observational, retrospective study in three primary care centres covering a population of 97,722 people. q Study population: patients with a prescription of any drug for osteoporosis. q Data collected were: age, gender, drugs for osteoporosis treatment and drugs that have a medium or high fall risk. RESULTS q 1,594 patients were treated with drugs for q Patients according to the number of drugs with osteoporosis falling risk concomitantly prescribed: 38.5% had Demographic data (n=1,594) one; 30.5% two; 17.9% three; 8.7% four and 4.4% five or more. Age – years* 72.4 ± 10.6 q Drugs for osteoporosis treatment are represented Female sex – n (%) 1,457 (91.5) in Figure 1. -

Pharmaco-Economic Study for the Prescribing of Prevention and Treatment of Osteoporosis
Technical Report 2: An analysis of the utilisation and expenditure of medicines dispensed for the prophylaxis and treatment of osteoporosis Technical report to NCAOP/HSE/DOHC By National Centre for Pharmacoeconomics An analysis of the utilisation and expenditure of medicines dispensed for the prophylaxis and treatment of osteoporosis February 2007 National Centre for Pharmacoeconomics Executive Summary 1. The number of prescriptions for the treatment and prophylaxis of osteoporosis has increased from 143,261 to 415,656 on the GMS scheme and from 52,452 to 136,547 on the DP scheme over the time period 2002 to 2005. 2. In 2005 over 60,000 patients received medications for the prophylaxis and treatment of osteoporosis on the GMS scheme with an associated expenditure of €16,093,676. 3. Approximately 80% of all patients who were dispensed drugs for the management of osteoporosis were prescribed either Alendronate (Fosamax once weekly) or Risedronate (Actonel once weekly) respectively. 4. On the DP scheme, over 27,000 patients received medications for the prophylaxis and treatment of osteoporosis in 2005 with an associated expenditure of € 6,028,925. 5. The majority of patients treated with drugs affecting bone structure were over 70 years e.g. 12,224 between 70 and 74yrs and 25,518 over 75yrs. 6. In relation to changes in treatment it was identified from the study that approximately 8% of all patients who are initiated on one treatment for osteoporosis are later switched to another therapy. 7. There was a statistically significant difference between the use of any osteoporosis medication and duration of prednisolone (dose response, chi- square test, p<0.0001). -

Role of Rhbmp-7, Fibronectin, and Type I Collagen in Dental Implant Osseointegration Process: an Initial Pilot Study on Minipig Animals
materials Article Role of rhBMP-7, Fibronectin, And Type I Collagen in Dental Implant Osseointegration Process: An Initial Pilot Study on Minipig Animals Gianmario Schierano 1,* , Rosa Angela Canuto 2, Mitzy Mauthe von Degerfeld 3 , Roberto Navone 4, Bruno Peirone 3, Giulio Preti 1 and Giuliana Muzio 2 1 Department of Surgical Science, C.I.R. Dental School, University of Torino, Via Nizza 230, 10126 Torino, Italy; [email protected] 2 Department of Clinical and Biological Sciences, University of Torino, Corso Raffaello 30, 10125 Torino, Italy; [email protected] (R.A.C.); [email protected] (G.M.) 3 Department of Veterinary Sciences, University of Torino, Largo Paolo Braccini 2, Grugliasco, 10095 Torino, Italy; [email protected] (M.M.v.D.); [email protected] (B.P.) 4 Department of Medical Science, University of Torino, Via Santena 5, 10126 Torino, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-(0)11-6331531-1532; Fax: +39-(0)11-6331513 Abstract: Background: The biological factors involved in dental implant osseointegration need to be investigated to improve implant success. Methods: Twenty-four implants were inserted into the tibias of six minipigs. Bone samples were obtained at 7, 14, and 56 days. Biomolecular analyses evaluated mRNA of BMP-4, -7, Transforming Growth Factor-β2, Interleukin-1β, and Osteocalcin in Citation: Schierano, G.; Canuto, R.A.; sites treated with rhBMP-7, Type 1 Collagen, or Fibronectin (FN). Inflammation and osteogenesis Mauthe von Degerfeld, M.; Navone, were evaluated by histological analyses. Results: At 7 and 14 days, BMP-4 and BMP-7 increased R.; Peirone, B.; Preti, G.; Muzio, G. -

Effect of Denosumab Or Alendronic Acid on the Progression of Aortic Stenosis: a Double-Blind Randomized Controlled Trial
10.1161/CIRCULATIONAHA.121.053708 Effect of Denosumab or Alendronic Acid on the Progression of Aortic Stenosis: A Double-Blind Randomized Controlled Trial Running Title: Pawade & Doris, et al.; Denosumab or Alendronic Acid for Aortic Stenosis Tania A. Pawade, MD1*; Mhairi K. Doris, MD1*; Rong Bing, MBBS1*; Audrey C. White1; Laura Forsyth, PhD2; Emily Evans, MSc3; Catriona Graham, MSc3; Michelle C. Williams, MD1; Edwin J.R. van Beek, MD4; Alison Fletcher, PhD2; Philip D. Adamson, MBChB1,5; Jack P.M. Andrews, MD1; Timothy R.G. Cartlidge, MD1; William S.A. Jenkins, MD1; Maaz Syed, MD1; Takeshi Fujisawa, PhD1; Christophe Lucatelli, PhD4; William Fraser, MBChB6; Stuart H. Ralston, MD7; Nicholas Boon, MD1; Bernard Prendergast, MD8; David E. Newby, MD1; Marc R. Dweck, MD1 1BHF Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, UK; 2Edinburgh Clinical Trials Unit, University of Edinburgh, Edinburgh, UK; 3Edinburgh Clinical Research Facility, University of Edinburgh, Edinburgh, UK; 4Edinburgh Imaging, University of Edinburgh, Edinburgh, UK; 5Christchurch Heart Institute, University of Otago, Christchurch, New Zealand; 6Norwich Medical School, University of East Anglia, Norwich, UK; 7Institute of Genetics and Molecular Medicine, University of Edinburgh, UK; 8King’s College London, London, UK Address for Correspondence Marc Dweck, MD BHF Centre for Cardiovascular Science Downloaded from http://ahajournals.org by on May 4, 2021 University of Edinburgh 47 Little France Crescent Edinburgh EH16 4TJ Email: [email protected] This article is published in its accepted form, it has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version. -

Bisphosphonates for Postmenopausal Osteoporosis
TITLE: Denosumab and Zoledronic Acid for Patients with Postmenopausal Osteoporosis: A Review of the Clinical Effectiveness, Safety, Cost Effectiveness, and Guidelines DATE: 11 September 2012 CONTEXT AND POLICY ISSUES Osteoporosis is characterized by low bone mineral density (BMD), deterioration of bone microarchitecture, and a consequent increase in bone fragility and risk of fracture. 1 Osteoporosis is most prevalent in postmenopausal women over 50 as estrogen levels decline. 2,3 The World Health Organization (WHO) estimates that 10% of 60 year old women, 20% of 70 year old Women, and 40% of 80 year old women worldwide have osteoporosis. 2 In Canada, postmenopausal osteoporosis affects more than 1.5 million women. 4 BMD is determined by the delicate balance of bone resorption (osteoclast activity) and bone formation (osteoblast activity), with osteoporosis occurring when bone resorption exceeds bone formation. 3 There are several therapies available for the prevention and management of postmenopausal osteoporosis. Nitrogen-containing bisphosphonates are highly potent inhibitors of osteoclastic bone resorption and have proven to be effective at reducing vertebral fracture risk. 5 Bisphosphonates such as alendronate and risedronate have been used for treatment of postmenopausal osteoporosis for many years and are taken orally with a daily dosage regimen. 5 Zoledronic acid (Aclasta) is a newer bisphosphonate administered intravenously once-yearly. 6 Recent advancements in the field of bone biology have led to the development of a new class of postmenopausal osteoporosis therapy. Denosumab (Prolia) is a human recombinant monoclonal antibody that binds to RANKL, a protein that acts as an essential mediator of osteoclast formation, thereby inhibiting osteoclast formation, function, and survival. -

Odanacatib, a Cathepsin K Inhibitor for the Treatment of Osteoporosis and Other Skeletal Disorders Associated with Excessive Bone Remodeling E Michael Lewiecki
!Drugs 200912(12):7~()-809 (~ Tliornso11 Reuters (Scientific) ltd ISSN 2040· 3410 DRUC PROFILE Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling E Michael Lewiecki Address New Mexico Clinic.ii Research & Osteoporosis Center, 300 Oak Street NE, Albuquerque, NM 87106, USA !:mail: lewiecki@aolcorn Odanacatib (MK·0822, MK-822) is an orally administered cathepsin K inhibitor being developed by Merck & Co Inc, under license from Ce/era Croup, for the treatment of osteoporosis and bone metastases. Cathepsin K, a lysosomol cysteine protease that is expressed by osteoclasts during the process of bone resorption, acts as the major col/agenase responsible for the degradation of the organic bone matrix during the bone remodeling process. Because excessive bone remodeling is a key element in the pathogenesis of postmenopousal osteoporosis and other skeletal disorders, cathepsin K is a potential target for therapeutic intervention. In a phase II clinical trial, weekly doses of odanacatib increased bone mineral density (BMD) and reduced bone turnover markers in postmenopausol women with low BMD. No tolerability concerns or evidence of skeletol toxicity were reported. Phase Ill trials, including a trial to evaluate the effects of odanocatib on froctt1re risk in up to 20,000 women with postmenopausol osteoporosis, were ongoing or recruiting participants at the time of publication. Odanacatib is a promising agent for the management of postmenopausal osteoporosis and other skeletal disorders associated with excessive bone remade/Ing. Introduction Therapeutic Odanacatib Osteoporosis is a common skeletal disease characterized by low bone mineral density (BMD) and poor bone quality Originator Celera Group that reduces bone strength and increases the risk of fractures [506066]. -

FOSAVANCE, INN-Alendronic Acid As Alendronate Sodium Trihydrate/Colecalciferol
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT FOSAVANCE 70 mg/2,800 IU tablets FOSAVANCE 70 mg/5,600 IU tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION FOSAVANCE 70 mg/2,800 IU tablets Each tablet contains 70 mg alendronic acid (as sodium trihydrate) and 70 micrograms (2,800 IU) colecalciferol (vitamin D3). Excipients with known effect Each tablet contains 62 mg lactose (as lactose anhydrous) and 8 mg sucrose. FOSAVANCE 70 mg/5,600 IU tablets Each tablet contains 70 mg alendronic acid (as sodium trihydrate) and 140 micrograms (5,600 IU) colecalciferol (vitamin D3). Excipients with known effect Each tablet contains 63 mg lactose (as lactose anhydrous) and 16 mg sucrose. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet FOSAVANCE 70 mg/2,800 IU tablets Modified capsule-shaped, white to off-white tablets, marked with an outline of a bone image on one side, and '710' on the other. FOSAVANCE 70 mg/5,600 IU tablets Modified rectangle-shaped, white to off-white tablets, marked with an outline of a bone image on one side, and '270' on the other. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications FOSAVANCE is indicated for the treatment of postmenopausal osteoporosis in women at risk of vitamin D insufficiency. It reduces the risk of vertebral and hip fractures. 4.2 Posology and method of administration Posology The recommended dose is one tablet once weekly. Patients should be instructed that if they miss a dose of FOSAVANCE they should take one tablet on the morning after they remember.