Sept. 26, 1967 K. F. WCKERT 3,343,908 METHOD OF REMOWING SULFUR TRIOXIDE FROM COMBUSTION GASES TO REDUCE THE CORROSIWE EFFECTS THEREOF Filed Feb. 6, 1964
Moo
90 'N | | | | | | | |N| | |
II (NNN | | || WIYN || 21 PSI 22d 3ad ared sad ea 20 agoo 920 /aeo Yao Mapo Moo
Za/2aaaaZ2/Ma Cl
INVENTOR At/A7 AAAD/WAWD W/CAEA77
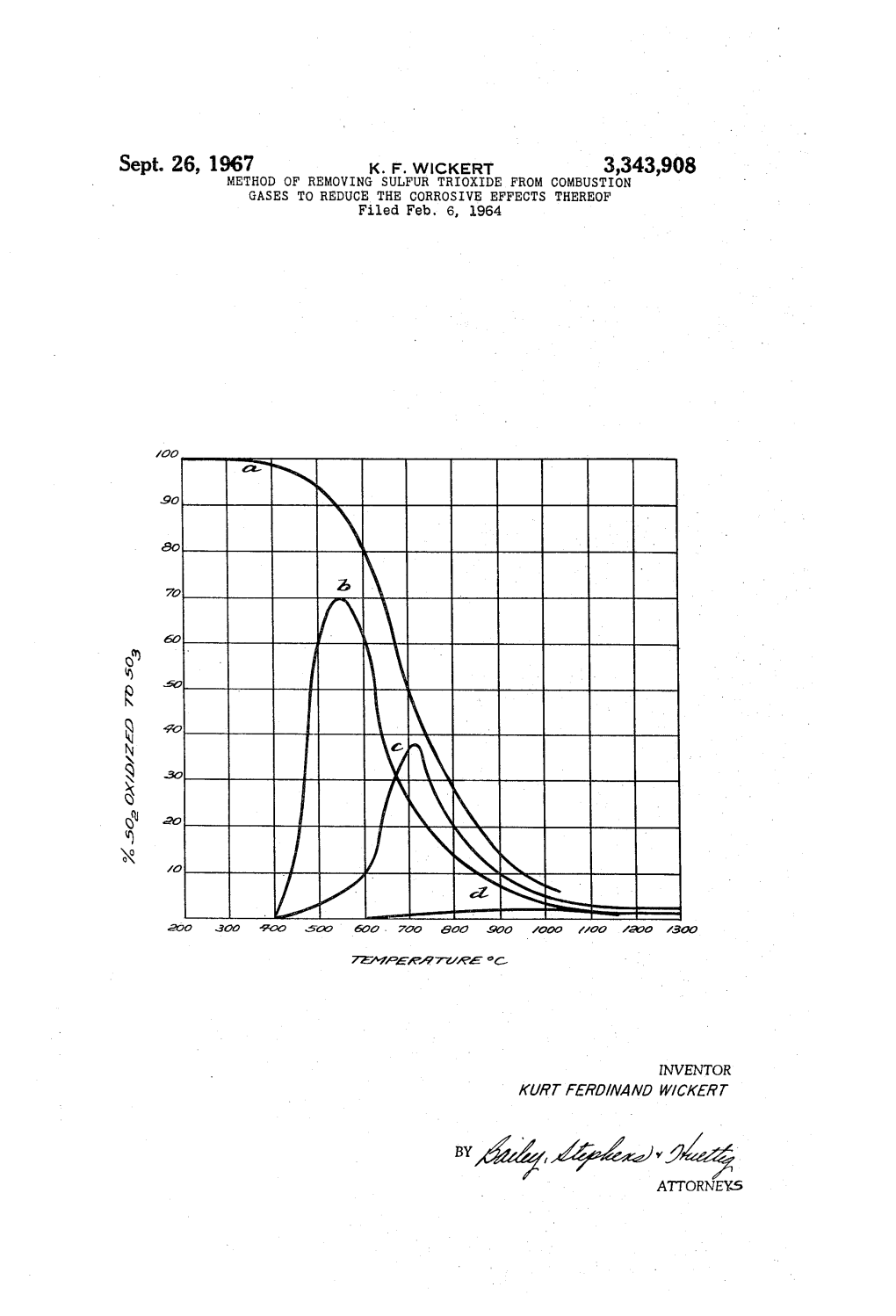
ATTORNEY.6 3,343,908 United States Patent Office Patented Sept. 26, 1967 2 3,343,908 According to the invention it was found that the oc METHOD OF REMOWING SULFUR TREOXDE currence of an acid dewpoint and the deposit of firmly FROM COMBUSTION GASES TO REDUCE adherent encrustations on the surfaces contacted with THE CORROSIVE EFFECTS THEREOF combustion gases in the lower temperature region could Kurt Ferdinand Wickert, Berlin-Siemensstadt, Germany, be practically completely avoided if a mixture of highly assignor to Deutsche Gold- und Silber-Scheideanstalt disperse finely divided basic and acidic oxides is uni vormals Roessler, Frankfurt an Main, Germany formly distributed in the combustion gases when they Filed Feb. 6, 1964, Ser. No. 342,953 Clains priority, application Germany, Feb. 13, 1963, have cooled down to about 400° C. Instead of basic D 40,866 oxides, carbonates or basic carbonates of elements form 10 ing such oxides can be used. Highly disperse silicon 4 Claims. (C. 23-2) dioxide or aluminum oxide or mixtures thereof have proved especially suited as the oxides of acidic character ABSTRACT OF THE DIS3LOSEURE which are used according to the invention and magne sium oxide or calcium oxide or mixtures thereof are Process for reducing dewpoint acid corrosion and 5 especially suited as the basic oxides. Magnesium carbon fouling of surfaces contacted at temperatures between ate or basic magnesium carbonate may also be used below about 400° C. and the ambient atmospheric tem with advantage in place of the magnesium oxide or cal perature with combustion gases containing sulfur tri cium oxide. It is essential for the success of the process oxide and water vapor comprising incoporating in a fine according to the invention that the highly disperse acidic state of distribution a mixture of (1) finely divided basic and basic materials employed are of sufficient fine grain material selected from the group consisting of calcium that their surface area measured by the BET method is oxide, magnesium oxide, magnesium carbonate, basic at least 30 m.2/g. Preferably, the surface area of the magnesium carbonate and mixtures thereof and (2) fine acidic oxides is larger, for example, about 100 m./g. ly divided acidic oxide material selected from the group The primary particle size of the acidic oxide, especially consisting of silicon dioxide, aluminum oxide and mix 25 of the silicon dioxide, should be under 150 mu, and pref tures thereof, said finely divided basic material and acidic erably be under 100 mu, oxide material having a surface area of at least 30 m./g., Under these conditions, practically all of the sulfur into such combustion gases when they have cooled to trioxide is bound in a very short period of time to mag a temperature of about 400° C., the molar proportion nesium sulfate or calcium sulfate, for example, in less of said acidic material being at least 0.5 mol per mol of : than 1 second. Magnesium oxide is especially suited as basic material calculated as oxide. the basic component of the oxide mixture as it practical ly does not react with sulfur dioxide. The quantities of the oxides employed insofar as The present invention relates to an improved process the basic component is concerned depends upon the for hindering acid dewpoint corrosion, particularly those quantity of sulfur trioxide present in the combustion caused by sulfuric acid, and fouling of equipment sub gases to be treated. The quantity of such basic oxide jected to combustion gases in the lower temperature should be stoichiometric with respect to sulfate forma region. tion. In order to insure prevention of the formation of It is known that liquid and solid fuels, such as oil and encrustations and solid deposits at least 0.5 mol of acidic coal, contain sulfur which in the combustion gases is 40 oxide should be employed per mol of alkaline oxide. partially oxidized to sulfur trioxide. Such sulfur trioxide The addition of the oxide mixture to the combustion forms sulfuric acid vapor at temperatures around 250 gases as indicated above should be at temperatures not C. with the water vapor which is always also present substantially above 400° C. as further formation of SO in the combustion gases and such sulfuric acid vapors, by the catalytic oxidation of SO practically does not depending upon their concentration in the combustion 45 occur under this temperature. gases, deposit at 180° C. and lower upon the available The accompanying drawing is a graph showing the surfaces of the equipment in contact with such combus equilibrium curves on the catalytic oxidiation of SO to tion gases. The strong corrosive effects of the deposition SO3 plotted against the temperature. of the sulfuric acid under some circumstances can even In such graph, curve a is the normal equilibrium be noticeable in the chimneys provided for exhausting 50 curve, whereas curves b and c give the amounts of the the cooled combustion gases to the atmosphere. In addi SO2 oxidiation with deposited oil ash b and with de tion, the sulfuric acid which may be carried along with posited coal fly ash c as the catalyst. Curve d corre the exhausted combustion gases under unfavorable weath sponds to the conversion of SO to SO3 in a quartz ap er conditions can cause considerable pollution of the paratus. As can be seen from these curves, if the addi atmosphere which can be disturbing to health, as well 55 tion of the oxides to the combustion gases is made when as plant growth, in the surrounding territory. Attempts the latter still have a temperature of 600° C., further have already been made to bind the sulfur trioxide con SO3 can be formed after the neutralization of the SO tained in the exhaust gases with magnesium oxide or present at such temperature with the stoichiometric magnesium compounds such as dolomite and thereby quantity of the basic oxide such as magnesium oxide and prevent any occurrence of an acid dewpoint. However, 60 as a consequence the further cooled gases will still con only unsatisfactory results have been attained thereby tain SO3 as the basic oxide was all consumed in the as it was only possible to shift the acid dewpoint to neutralization of the SO present at 600° C. somewhat lower temperatures. Previously, attempts to Evidently the effect of the acidic oxide in the oxide eliminate the more or less strong fouling caused by de mixture employed according to the invention is that posits of solids have not been successful to any degree 65 the finely divided oxide with large surface area prevents worth mentioning. To the contrary. the deposits of the the formation of firmly adhering encrustations which substances added to reduce the dewpoint which have would have been produced from the magnesium sulfate partially been converted to sulfates produce hard firmly by the action of the water vapor contained in the com adhering crusts, for example, in the low temperature bustion gases through interstratification. region of the boiler tubes and the plates of the air pre 70 The introduction of the oxide mixture into the com heaters, which cannot be removed with the usual means bustion gases can be effected at a suitable location at without interrupting the operation. which the combustion gases have cooled to about 400° 3,343,908 3. 4. C. by blowing such mixture in solid form as a dust. 10 vol-percent water vapor is at a temperature of 40 However, according to an advantageous embodiment of 45 C. it raises up to 150-180° C., if there is a small the invention, such oxide mixture is introduced into the content of SO3 in such gas-mixture. combustion gases in the form of an aqueous dispersion. SiO2 was obtained by the hydrolysis in the gas phase For this purpose the disperse oxide mixture, is first dis 5 in the pyrogenic manner, the primary particle size was persed in water and such dispersion blown into the com about 0.005-0.025u, the specific surface according to bustion gases with the aid of an injector or other suit the BET method 190 m./g. The MgO had a specific able device. The aqueous dispersing agent vaporizes surface of about 50 m./g. and consisted of scaly agglom quickly at the 400° C. temperature, leaving a uniformly erates up to 3p size. distributed oxide smoke which effectively binds the sul Analogical results will be obtained by replacing of fur trioxide chemically and through the presence of the O silicon dioxide in the whole or partly by titanium oxide acidic oxide effectively prevents the formation of en and magnesium oxide in the whole or partly by calcium crustations. oxide or basic or neutral magnesium carbonate. In view of the fine particle size and large surface area I claim: of the oxides employed according to the invention, they 1. A process for reducing dewpoint acid corrosion are easily converted to stable aqueous dispersion. In the and fouling of surfaces contacted at temperatures be System of magnesium oxide and water, a solids concen tween below about 400 C. and the ambient atmospheric tration of about 8% by weight should be selected where temperature with combustion gases containing sulfur as in the system of silicon dioxide and water it can even trioxide and water vapor comprising incorporating in a be about 20% by weight. A 1:1 mixture of silicon di 20 fine state of distribution a mixture of (1) finely divided oxide and magnesium oxide can still be processed to a basic material selected from the group consisting of cal suitable dispersion at a 10% by weight solids content. cium oxide, magnesium oxide, magnesium carbonate, basic magnesium carbonate and mixtures thereof and Example (2) finely divided acidic oxide material selected from A boiler of a power station needs 10 t./h. of fuel oil the group consisting of silicon dioxide, aluminum oxide which a below heating value of 9,600 kcal./kg. The ex and mixtures thereof, said finely divided basic and acidic cess air is 10%. The oil has a sulfur content of 3.0% oxide materials having a surface area of at least 30 in the average. m./g., into such combustion gases when they have 1 kg. of fuel oil generates at burning with 10% of air cooled to a temperature of about 400° C., the molar excess a smoke gas volume of 12.4 Nm.3. 10 t of fuel 30 proportion of said acidic material being at least 0.5 mol oil generate under the same conditions a waste gas per mol of basic material calculated as oxide. amount of 124,000 Nm.3. This waste gas contains 600 2. The process of claim 1 in which said basic material kg. of SO2 if the whole sulfur would be burned to SO. is finely divided magnesium oxide having a surface area The Waste gas examination showed that the thirtieth of about 30 m./g. and said acidic oxide is silicon di part of this SO2 amount consists of SO3, that means that 3 5 oxide having a surface area of about 100 m.2/g. 20 kg. of SO were transformed into 25 kg. of SO3. 3. The process of claim 1 in which the quantity of These 25 kg. of SO3 will be neutralized by blowing in basic material incorporated in the combustion gases of MgO. Theoretically 12.5 kg. of MgO will be needed is substantially stoichiometric for the formation of the for this. It has been practically worked with an MgO corresponding sulfate with the sulfur trioxide contained excess of 10%. On a spot where the smoke gas stream 40 in such combustion gases. had a temperature of about 400, a mixture of 14.0 kg. 4. The process of claim 1 in which said mixture of of MgO and 15 kg. of SiO was blown in per hour. By finely divided basic material and finely divided acidic the treatment of the waste gas according to the invention oxide material is supplied to the combustion gases in the content of 25 kg. of SOs in 124,000 Nm.3 of waste the form of an aqueous dispersion. gas (corresponding to 20 kg. of SO2) was reduced to 0.37 45 kg. of SO3 or 0.0003 g. SO/Nm.3 of waste gas. This References Cited SO concentration will no more effect a raising of the UNITED STATES PATENTS water vapor dewpoint, that means an acid dewpoint 2,718,453 9/1955. Beckman is not anymore demonstrable. The Water vapor dewpoint of a waste gas containing EARL C. THOMAS, Primary Examiner.