Pdf 918.71 K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government of India United States Agency for International Development World Vision
Government of India United States Agency for International Development World Vision THIRD ANNUAL REVIEW REPORT Ballia Rural Integrated Child Survival Project Uttar Pradesh, India USAID Grant # FAO – 00 – 98 – 00041 – 00 January 31, 2002 Beginning Date : October 1, 1998 Ending Date : September 30, 2002 Submitted to Child Survival Grant Program USAID/BHR/PVC PVO Field Office : PVO Headquarters: K.A.Jayakumar David Grosz, MPH World Vision India WVUS Program Officer for India ADP Ballia World Vision Inc P.O.Box 25 34834 Weyerhaeuser Way South Harpur, Ballia, U.P 277 001 Federal Way, Washington 98063 Phone: (91) 54 982 3014 Phone: 253/815-2092 Fax : (91) 54 982 3014 Fax : 253/815-3424 email: [email protected] email [email protected] TABLE OF CONTENTS LIST OF APPENDICES 4 LIST OF ACRONYMS 5 1. EXECUTIVE SUMMARY 6 2. INTRODUCTION 8 3. EXPECTATIONS OF THE THIRD ANNUAL REVIEW 9 4. METHODOLOGY FOR THE THIRD ANNUAL REVIEW 10 5. PROJECT BACKGROUND 12 6. MODELS OF REPLICATION 14 7. CAPACITY BUILDING: 16 8. SUSTAINABILITY 19 9. MAIN ACCOMPLISHMENTS 22 PREVENTION OF MALNUTRITION & VITAMIN A DEFICIENCY 22 INCREASED COVERAGE OF IMMUNIZATION 24 DIARRHEA AND PNEUMONIA CASE MANAGEMENT 26 BIRTH SPACING 27 ESSENTIAL CARE OF THE NEWBORN 29 DEVELOPMENT OF BASELINES FOR THE DISTRICT 29 10. SUPPORT SYSTEMS 30 MANAGEMENT 30 HUMAN RESOURCE 31 HEALTH MANAGEMENT INFORMATION SYSTEM 32 FINANCE 34 11. ISSUES IDENTIFIED BY THE MTE AND THE PROJECT RESPONSE 36 12. CHALLENGES AND CONSTRAINTS 39 ADP Ballia Third Annual Review Report, October 2001 Page No. 2 13. CHANGES IN THE PROJECT DESIGN 40 14. -

National Ganga River Basin Authority (Ngrba)
NATIONAL GANGA RIVER BASIN AUTHORITY (NGRBA) Public Disclosure Authorized (Ministry of Environment and Forests, Government of India) Public Disclosure Authorized Environmental and Social Management Framework (ESMF) Public Disclosure Authorized Volume I - Environmental and Social Analysis March 2011 Prepared by Public Disclosure Authorized The Energy and Resources Institute New Delhi i Table of Contents Executive Summary List of Tables ............................................................................................................... iv Chapter 1 National Ganga River Basin Project ....................................................... 6 1.1 Introduction .................................................................................................. 6 1.2 Ganga Clean up Initiatives ........................................................................... 6 1.3 The Ganga River Basin Project.................................................................... 7 1.4 Project Components ..................................................................................... 8 1.4.1.1 Objective ...................................................................................................... 8 1.4.1.2 Sub Component A: NGRBA Operationalization & Program Management 9 1.4.1.3 Sub component B: Technical Assistance for ULB Service Provider .......... 9 1.4.1.4 Sub-component C: Technical Assistance for Environmental Regulator ... 10 1.4.2.1 Objective ................................................................................................... -

For Design, Development & Implementation of Web Based
U.P. Electronics Corporation Limited (U.P. Government Undertaking) 10 Ashok Marg, Lucknow-226001 Telephones : (0522) 2286808, 2286809, 4130303 Fax : (0522) 2288583 Website : www.uplc.in E-mail : [email protected], [email protected] Request for Proposal (RFP) For Design, Development & Implementation of Web based Application Software & Web Portal for Bagh Sanrakshan Samit, Uttar Pradesh, Lucknow (UP). Bid Reference : UPLC/Software / 2014-15/001 E-tender0 Portal : http://etender.up.nic.in Critical Dates SN Particulars Date Time 1 Publishing Date 31 May 2014 06:55 PM 2 Bid Submission Start Date 31 May 2014 6:55 PM Onwards 3 Bid Submission End Date 09 Jun 2014 5:00 PM 4 Bid Opening Date 11 Jun 2014 05:10 PM Place of Opening e-Bids : UP Electronics Corporation Ltd 10-Ashok Marg, Lucknow-226001 This Document Contains – 32 pages Page 1 of 31 Index Page SN Contents Nos 1 e-Bid Notice 3 2 INTRODUCTION [about Bagh Sanrakshan Samit, Uttar Pradesh, Lucknow Lucknow ] 5 3 SECTION I : Letter of Invitation 4 SECTION II: INSTRUCTIONS TO BIDDERS (ITB) 5 SECTION III: TERMS OF REFERENCE (TOR) AND SCOPE OF WORK 6 SECTION IV: BIDDER'S ELIGIBILITY CRITERIA 7 SECTION V – STANDARD TERMS AND CONDITIONS 8 SECTION VI- TECHNICAL PROPOSAL SUBMISSION FORM (Annexure I & II) 9 DECLARATION FOR PROPOSAL SUBMISSION FORM (Annexure III) 10 FINANCIAL PROPOSAL SUBMISSION FORM (Annexure IV) Page 2 of 31 e-Bid Notice FOR For Design, Development & Implementation of Web based Application Software & Web Portal for Bagh Sanrakshan Samit, Uttar Pradesh, Lucknow (UP). Online e-bids are invited from experienced Service Providers, who are already empanelled with U.P. -

Asian Ibas & Ramsar Sites Cover
■ INDIA RAMSAR CONVENTION CAME INTO FORCE 1982 RAMSAR DESIGNATION IS: NUMBER OF RAMSAR SITES DESIGNATED (at 31 August 2005) 19 Complete in 11 IBAs AREA OF RAMSAR SITES DESIGNATED (at 31 August 2005) 648,507 ha Partial in 5 IBAs ADMINISTRATIVE AUTHORITY FOR RAMSAR CONVENTION Special Secretary, Lacking in 159 IBAs Conservation Division, Ministry of Environment and Forests India is a large, biologically diverse and densely populated pressures on wetlands from human usage, India has had some country. The wetlands on the Indo-Gangetic plains in the north major success stories in wetland conservation; for example, of the country support huge numbers of breeding and wintering Nalabana Bird Sanctuary (Chilika Lake) (IBA 312) was listed waterbirds, including high proportions of the global populations on the Montreux Record in 1993 due to sedimentation problem, of the threatened Pallas’s Fish-eagle Haliaeetus leucoryphus, Sarus but following successful rehabilitation it was removed from the Crane Grus antigone and Indian Skimmer Rynchops albicollis. Record and received the Ramsar Wetland Conservation Award The Assam plains in north-east India retain many extensive in 2002. wetlands (and associated grasslands and forests) with large Nineteen Ramsar Sites have been designated in India, of which populations of many wetland-dependent bird species; this part 16 overlap with IBAs, and an additional 159 potential Ramsar of India is the global stronghold of the threatened Greater Sites have been identified in the country. Designated and potential Adjutant Leptoptilos dubius, and supports important populations Ramsar Sites are particularly concentrated in the following major of the threatened Spot-billed Pelican Pelecanus philippensis, Lesser wetland regions: in the Qinghai-Tibetan plateau, two designated Adjutant Leptoptilos javanicus, White-winged Duck Cairina Ramsar Sites overlap with IBAs and there are six potential scutulata and wintering Baer’s Pochard Aythya baeri. -
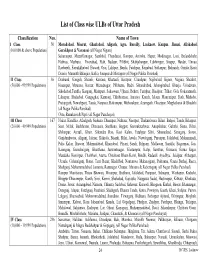
List of Class Wise Ulbs of Uttar Pradesh
List of Class wise ULBs of Uttar Pradesh Classification Nos. Name of Town I Class 50 Moradabad, Meerut, Ghazia bad, Aligarh, Agra, Bareilly , Lucknow , Kanpur , Jhansi, Allahabad , (100,000 & above Population) Gorakhpur & Varanasi (all Nagar Nigam) Saharanpur, Muzaffarnagar, Sambhal, Chandausi, Rampur, Amroha, Hapur, Modinagar, Loni, Bulandshahr , Hathras, Mathura, Firozabad, Etah, Badaun, Pilibhit, Shahjahanpur, Lakhimpur, Sitapur, Hardoi , Unnao, Raebareli, Farrukkhabad, Etawah, Orai, Lalitpur, Banda, Fatehpur, Faizabad, Sultanpur, Bahraich, Gonda, Basti , Deoria, Maunath Bhanjan, Ballia, Jaunpur & Mirzapur (all Nagar Palika Parishad) II Class 56 Deoband, Gangoh, Shamli, Kairana, Khatauli, Kiratpur, Chandpur, Najibabad, Bijnor, Nagina, Sherkot, (50,000 - 99,999 Population) Hasanpur, Mawana, Baraut, Muradnagar, Pilkhuwa, Dadri, Sikandrabad, Jahangirabad, Khurja, Vrindavan, Sikohabad,Tundla, Kasganj, Mainpuri, Sahaswan, Ujhani, Beheri, Faridpur, Bisalpur, Tilhar, Gola Gokarannath, Laharpur, Shahabad, Gangaghat, Kannauj, Chhibramau, Auraiya, Konch, Jalaun, Mauranipur, Rath, Mahoba, Pratapgarh, Nawabganj, Tanda, Nanpara, Balrampur, Mubarakpur, Azamgarh, Ghazipur, Mughalsarai & Bhadohi (all Nagar Palika Parishad) Obra, Renukoot & Pipri (all Nagar Panchayat) III Class 167 Nakur, Kandhla, Afzalgarh, Seohara, Dhampur, Nehtaur, Noorpur, Thakurdwara, Bilari, Bahjoi, Tanda, Bilaspur, (20,000 - 49,999 Population) Suar, Milak, Bachhraon, Dhanaura, Sardhana, Bagpat, Garmukteshwer, Anupshahar, Gulathi, Siana, Dibai, Shikarpur, Atrauli, Khair, Sikandra -

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Advocates in District Court, Ballia
Advocates in District Court, Ballia S.No. Name Father's Name Registration No. Address JUTHI TIWARI KE TOLA, ACHLGARH, BALLIA 1 RAM NIWASH TIWARI LATE BRIJNATH TIWARI UP1714/1987 ### U.P. VILL-PANDEYPUR, P.O. TAKHA, DISTT 2 SHIV KRIPA PANDEY NARENDRA NATH PANDEY UP5618/2006 ### BALLIA, UP VILL SAEMPUR, POST ISARI SALEMPUR, AKHILESH KUMAR SINGH LATE BHAGWATI SHARAN SINGH UP1541/1995 ### 3 BALLIA 4 PRASHANT MISHRA RAMA SHANKER MISHRA UP7913/2001 ADHIWAKTA NAGAR BALLIA ### 5 RAMASHANKAR MISHRA LATE BALBHADRA MISHRA UP3145/1995 ADVOCATE COLONY, BALLIA ### 6 ATUL KUMAR SRIVASTAVA DAYA NAND SRIVASTAVA UP3489/1992 YADAV NIVAS KOTWALI HARPUR BALLIA UP### VILL POST SAGARPALI PHEPHNA DISTT RAJIV KUMAR SRIVASTAVA LATE RAJENDRA PRASAD UP2276/2005 ### 7 BALLIA VUILL MAHUWEE POST SHIVPUR GANESH JI PANDEY LATE RAM LAL PANDEY UP3374/2013 ### 8 (DATTIWAR) DISTT BALLIA UP MOHALLA MILKI, POST SIKANDERPUR, SARWAT MOID LATE ABDUL NOID UP616/1986 ### 9 DISTT BALLIA UP VILL- SHUKLA CHHAPRA POST – 10 ARUN KUMAR SHUKLA LATE RAM KRIPAL SHUKLA UP679/1987 ### MAJHAUWAN , BALLIA 11 MANOJ KUMAR SINGH AWADHESH KUMAR SINGH UP4167/1996 VILL – GAYGHAT VIA- REOTI BALLIA ### VILL – KUMHAILA POLICE STATION – 12 VIRENDRA KUMAR SINGH LATE RAJ KISHORE SINGH UP4084/1986 ### SHUKHPURA , BALLIA 13 HARINDRA NATH SINGH LAET SHIV DATT SINGH UP666/1998 VILL – APAIL, APAIL, BALLIA ### 14 SANJEEV KUMAR RAI LATE MADAN GOPAL RAI UP11933/1999 VILL – NARHI , POST – NARHI, BALLIA ### MOHALLA – ANAND NAGAR , NAI BASTI , 15 PREM KUMAR SHUKLA LATE VACHASPATI SHUKLA UP17604/1999 ### BALLIA 16 -

A Block Wise Study, Faizabad District Sadaf and Abdul Munir Regional Development Is a Multi-Dimensional Phenomenon
National Geographical Journal of India, An International Peer-reviewed and refereed Journal of India (NGSI-BHU, ISSN: 0027-9374/2015/1564), vol. 61 (4), Dec. : 321-332 Spatial Analysis of Regional Development : A Block Wise Study, Faizabad District Sadaf and Abdul Munir Regional development is a multi-dimensional phenomenon. It represents an overall development of any region. The concept of regional development means the fullest development of any region according to its potentialities. The problem of regional disparities is not found in India only rather it is a global problem. However, in India, it is more common than in any other country. The present paper is an attempt to show the spatial pattern of regional development at block level of Faizabad district for the period of 2010-2011. Twenty two variables have been selected for analyzing the spatial variation of development of 11 blocks of the district. For this study, secondary data have been collected from the stastical bulletin and primary census abstract. Composite index of development and Z score have been used to calculate the agricultural development, infrastructural development, industrial development, socio-economic development and finally the level of overall development. The analysis revealed that Amaniganj holds the first position while Rudauli attains the low level of development. Keywords : Regional development, Z score, Composite index of development Introduction development normally begins with identification Regional development is a multi- and analysis of regional disparities.” dimensional concept. It represents the Identification of regional disparities is very integrated study of social, economic, important in making the plan for the agricultural, infrastructure and industrial development with sustainability. -

Infected Areas As on 3 August 1972 — Zones Infectées Au 3 Août 1972
— 304 SMALLPOX (amuL) - VARIOLE (suite) c D C D Africa (conid.) — Afrique (suite) INDIA — INDE 23-29. YU PAKISTAN 18-24, VI W est P akistan C D Calcutta (P) (excl. A ). 4 6 SUDAN — SOUDAN 23-29.VH North-West Frontier Province 9-15. VII D istricts Bahr el Ghasal Province Andhra Pradesh State Wau Rur. C................. 2 ... Bannu .................... 6 1 Hyderabad D. 5 0 Dera Ismail Khan . 12 3 Kassala Province K o h a t .................... 1 0 Madhya Pradesh State Aroma Rur, C. 6 ... Peshawar ..... 9 0 17 6 Tikamgarh D ............... c D c D 25.VI-1.VH 2-8. VU Asia — Asie Maharashtra State Lahore (excl. A) . 28 1 3 0 c D C D Thana D ....................... 12 5 W est P akistan BANGLADESH 2-8.VU 9-15.VH Mysore State North-West Frontier Province Dacca Division 2 Bannu D ................... 0 1 0 1 Dacca D ................... 0 0 1 0 Bidar D ........................ 0 1 1 Peshawar D. 27 1 17 1 Faridpur D ............... 66 6 65 23 Gulbarga D.................. Punjab Province Khulna Division Uttar Pradesh State D istricts D istricts D istricts Gujranwala .... 24 3 1 0 Bakerganj (Barisal). 98 15 75 44 B ah raic h ............... ... 48 9 Muzaffargarh . 0 0 1 0 Jessore ................... 42 2 9 0 Ballia ....................... 0 1 Rahim Yar Khan. 1 0 5 1 Khulna ................ 36 3 43 11 Bareilly................... - 16 1 S ia lk o t.................... 0 0 6 0 Patuakhali .... 0 0 8 5 Kberi ....................... 12 1 11-17.VI1 Rajshahi Division M ain p u ri.................. -

Uttar Pradesh BSAP
NATIONAL BIODIVERSITY STRATEGY AND ACTION PLAN, UTTAR PRADESH (U.P.) Coordinator Coordinated by: U. Dhar GBPIHED TEAM S.S. Samant Asha Tewari R.S. Rawal NBSAP, U.P. Members Dr. S.S. Samant Dr. B.S. Burphal DR. Ipe M. Ipe Dr. Arun Kumar Dr. A.K. Singh Dr. S.K. Srivastava Dr. A.K. Sharma Dr. K.N. Bhatt Dr. Jamal A. Khan Miss Pia Sethi Dr. Satthya Kumar Miss Reema Banerjee Dr. Gopa Pandey Dr. Bhartendu Prakash Dr. Bhanwari Lal Suman Dr. R.D. Dixit Mr. Sameer Sinha Prof. Ajay S. Rawat 1 Contributors B.S. Burphal Pia Sethi S.K. Srivastava K.N. Bhatt D.K. pande Jamal A. Khan A.K. Sharma 2 CONTENTS CHAPTER 1. INTRODUCTION 1.1 . Brief background of the SAP 1.2 . Scope of the SAP 1.3 . Objectives of the SAP 1.4 . Contents of the SAP 1.5 . Brief description of the SAP CHAPTER 2. PROFILE OF THE AREA 2.6 . Geographical profile 2.7 . Socio- economic profile 2.8 . Political profile 2.9 . Ecological profile 2.10.Brief history CHAPTER 3. CURRENT (KNOWN) RANGE AND STATUS OF BIODIVERSITY 3.1. State of natural ecosystems and plant / animal species 3.2. State of agricultural ecosystems and domesticated plant/ animal species CHAPTER 4. STATEMENTS OF THE PROBLEMS RELATED TO BIODIVERSITY 4.1. Proximate causes of the loss of biodiversity 4.2. Root causes of the loss of biodiversity CHAPTER 5. MAJOR ACTORS AND THEIR CURRENT ROLES RELEVANT TO BIODIVERSITY 5.1. Governmental 5.2. Citizens’ groups and NGOs 5.3. Local communities, rural and urban 5.4. -
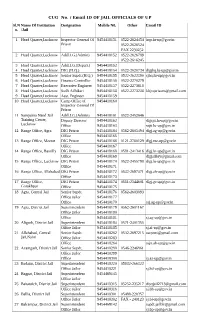
CUG No. / Email ID of JAIL OFFICIALS of up Sl.N Name of Institution Designation Mobile N0
CUG No. / Email ID OF JAIL OFFICIALS OF UP Sl.N Name Of Institution Designation Mobile N0. Other Email ID o. /Jail 1 Head Quarter,Lucknow Inspector General Of 9454418151 0522-2624454 [email protected] Prison 0522-2626524 FAX 2230252 2 Head Quarter,Lucknow Addl.I.G.(Admin) 9454418152 0522-2626789 0522-2616245 3 Head Quarter,Lucknow Addl.I.G.(Depart.) 9454418153 4 Head Quarter,Lucknow DIG (H.Q.) 9454418154 0522-2620734 [email protected] 5 Head Quarter,Lucknow Senior Supdt.(H.Q.) 9454418155 0522-2622390 [email protected] 6 Head Quarter,Lucknow Finance Controller 9454418156 0522-2270279 7 Head Quarter,Lucknow Executive Engineer 9454418157 0522-2273618 8 Head Quarter,Lucknow Sodh Adhikari 9454418158 0522-2273238 [email protected] 9 Head Quarter,Lucknow Asst. Engineer 9454418159 10 Head Quarter,Lucknow Camp Office of 9454418160 Inspector General Of Prison 11 Sampurna Nand Jail Addl.I.G.(Admin) 9454418161 0522-2452646 Training Center, Deputy Director 9454418162 [email protected] Lucknow Office 9454418163 [email protected] 12 Range Office, Agra DIG Prison 9454418164 0562-2605494 [email protected] Office 9454418165 13 Range Office, Meerut DIG Prison 9454418166 0121-2760129 [email protected] Office 9454418167 14 Range Office, Bareilly DIG Prison 9454418168 0581-2413416 [email protected] Office 9454418169 [email protected] 15 Range Office, Lucknow DIG Prison 9454418170 0522-2455798 [email protected] Office 9454418171 16 Range Office, Allahabad DIG Prison 9454418172 0532-2697471 [email protected] Office 9454418173 17 Range Office, DIG Prison 9454418174 0551-2344601 [email protected] Gorakhpur Office 9454418175 18 Agra, Central Jail Senior Supdt. -

Agroforestry Practices in Ballia District of Eastern Plain Region of Uttar
International Journal of Environmental & Agriculture Research (IJOEAR) ISSN:[2454-1850] [Vol-6, Issue-1, January- 2020] Agroforestry Practices in Ballia District of Eastern Plain Region of Uttar Pradesh, India Hari Om Shukla1, Anita Tomar2, Amit Kushwaha3, Rajeev Singh4*, Anubha Srivastav5 Forest Research Centre for Eco-rehabilitation, Prayagraj 4*Krishi Vigyan Kendra, Ballia Abstract— Agroforestry is an efficient land-use system where trees or shrubs are grown with arable crops, seeking positive interactions in enhancing productivity on the sustainable basis. Agroforestry combines agriculture and forestry technologies to create more integrated, diverse, productive, profitable, healthy and sustainable land-use systems. The study was conducted in selected villages (1%) of Ballia District of Eastern plain region of Uttar Pradesh in India during the year 2018 to record the crop combinations with tree species and their stratified arrangement to identify agroforestry practices. The socio-economic studies based on general village profile, land holding, land use pattern and tree species planting pattern were performed in 1 % villages to collect the data with structured questionnaire and Participatory Rural Appraisal (PRA) tools. The results demonstrated that a total of six different agroforestry practices, agri-silviculture, silvi-horticulture, agri- horticulture, agri-silvi-horticulture, silvi-pastoral, and homestead existed in different villages. Out of different categories, timber, fruits, medicinal, agriculture, flower and other plant species were recorded. It was recorded that out of existing agroforestry practices, scattered near farms and around homestead was found most common (about 37.7 %) followed by agri-silviculture (20.20 %), silvi-horticulture (19.1 %) and agri-horticulture (12.3 %). The pattern of plantation on bunds and blocks was 17.94 % and 16.82 % respectively.