The Early Differentiation History of Mars from New Constraints on 182W-142Nd Isotope
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Origin of Ureilites Inferred from a SIMS Oxygen Isotopic and Trace Element Study of Clasts in the Dar Al Gani 319 Polymict Ureilite
Geochimica et Cosmochimica Acta, Vol. 68, No. 20, pp. 4213-4235, 2004 Copyright © 2004 Elsevier Ltd Pergamon Printed in the USA. All rights reserved 0016-7037/04 $30.00 ϩ .00 doi:10.1016/j.gca.2004.03.020 Origin of ureilites inferred from a SIMS oxygen isotopic and trace element study of clasts in the Dar al Gani 319 polymict ureilite 1,†, 2 1 1,‡ 1 3,4 NORIKO T. KITA, *YUKIO IKEDA, SHIGEKO TOGASHI, YONGZHONG LIU, YUICHI MORISHITA, and MICHAEL K. WEISBERG 1Geological Survey of Japan, AIST, AIST Central 7, Tsukuba 305-8567, Japan 2Faculty of Science, Ibaraki University, Mito 301-8512, Japan 3Department of Physical Sciences, Kingsborough College (CUNY), 2001 Oriental Boulevard, Brooklyn, NY 11235, USA 4Department of Earth and Planetary Sciences, American Museum of Natural History, New York, NY 10024, USA (Received June 12, 2003; accepted in revised form March 3, 2004) Abstract—Secondary ion mass spectrometer (SIMS) oxygen isotope analyses were performed on 24 clasts, representing 9 clast types, in the Dar al Gani (DaG) 319 polymict ureilite with precisions better than 1‰. Olivine-rich clasts with typical ureilitic textures and mineral compositions have oxygen isotopic compositions that are identical to those of the monomict ureilites and plot along the CCAM (Carbonaceous Chondrite Anhydrous Mineral) line. Other igneous clasts, including plagioclase-bearing clasts, also plot along the CCAM line, indicating that they were derived from the ureilite parent body (UPB). Thus, we suggest that some of the plagioclase-bearing clasts in the polymict ureilites represent the “missing basaltic component” produced by partial melting on the UPB. -

Mineralogy and Petrology of the Angrite Northwest Africa 1296 Albert Jambon, Jean-Alix Barrat, Omar Boudouma, Michel Fonteilles, D
Mineralogy and petrology of the angrite Northwest Africa 1296 Albert Jambon, Jean-Alix Barrat, Omar Boudouma, Michel Fonteilles, D. Badia, C. Göpel, Marcel Bohn To cite this version: Albert Jambon, Jean-Alix Barrat, Omar Boudouma, Michel Fonteilles, D. Badia, et al.. Mineralogy and petrology of the angrite Northwest Africa 1296. Meteoritics and Planetary Science, Wiley, 2005, 40 (3), pp.361-375. hal-00113853 HAL Id: hal-00113853 https://hal.archives-ouvertes.fr/hal-00113853 Submitted on 2 May 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Meteoritics & Planetary Science 40, Nr 3, 361–375 (2005) Abstract available online at http://meteoritics.org Mineralogy and petrology of the angrite Northwest Africa 1296 A. JAMBON,1 J. A. BARRAT,2 O. BOUDOUMA,3 M. FONTEILLES,4 D. BADIA,1 C. GÖPEL,5 and M. BOHN6 1Laboratoire Magie, Université Pierre et Marie Curie, CNRS UMR 7047, case 110, 4 place Jussieu, 75252 Paris cedex 05, France 2UBO-IUEM, CNRS UMR 6538, Place Nicolas Copernic, F29280 Plouzané, France 3Service du MEB, UFR des Sciences de la Terre, Université Pierre et Marie Curie, case 110, 4 place Jussieu, 75252 Paris cedex 05, France 4Pétrologie, Modélisation des Matériaux et Processus, Université Pierre et Marie Curie, case 110, 4 place Jussieu, 75252 Paris cedex 05, France 5Laboratoire de Géochimie et Cosmochimie, Institut de Physique du Globe, CNRS UMR 7579, 4 place Jussieu, 75252 Paris cedex 05, France 6Ifremer-Centre de Brest, CNRS-UMR 6538, BP70, 29280 Plouzané Cedex, France *Corresponding author. -

Design of Low-Altitude Martian Orbits Using Frequency Analysis A
Design of Low-Altitude Martian Orbits using Frequency Analysis A. Noullez, K. Tsiganis To cite this version: A. Noullez, K. Tsiganis. Design of Low-Altitude Martian Orbits using Frequency Analysis. Advances in Space Research, Elsevier, 2021, 67, pp.477-495. 10.1016/j.asr.2020.10.032. hal-03007909 HAL Id: hal-03007909 https://hal.archives-ouvertes.fr/hal-03007909 Submitted on 16 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Design of Low-Altitude Martian Orbits using Frequency Analysis A. Noulleza,∗, K. Tsiganisb aUniversit´eC^oted'Azur, Observatoire de la C^oted'Azur, CNRS, Laboratoire Lagrange, bd. de l'Observatoire, C.S. 34229, 06304 Nice Cedex 4, France bSection of Astrophysics Astronomy & Mechanics, Department of Physics, Aristotle University of Thessaloniki, GR 541 24 Thessaloniki, Greece Abstract Nearly-circular Frozen Orbits (FOs) around axisymmetric bodies | or, quasi-circular Periodic Orbits (POs) around non-axisymmetric bodies | are of primary concern in the design of low-altitude survey missions. Here, we study very low-altitude orbits (down to 50 km) in a high-degree and order model of the Martian gravity field. We apply Prony's Frequency Analysis (FA) to characterize the time variation of their orbital elements by computing accurate quasi-periodic decompositions of the eccentricity and inclination vectors. -
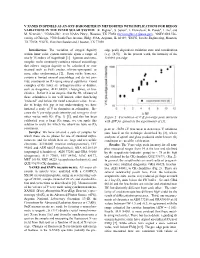
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Cosmic-Ray-Exposure Ages Martian Meteorites Million Years
Introduction to MMC 2012 someone keeps adding pieces as you go along (see my Introduction 2006). Most Martian meteorites are mafic As of the end of 2012, we now know of about 65 basalts. That is, they have an abundance of Fe and different meteorites from Mars with a total weight about Mg, are low in Si and Al and have a texture of 120 kilograms. Some fell as showers (e.g. Nakhla, intergrown minerals typical of terrestrial basalts (the Tissint), and some can be paired by common lithology, tradition has been to call them “shergottites”, after the age and fall location, but it is more instructive to first sample recognized as such). Some often have an consider how they are grouped by “launch pairs”. If abundance of large olivine crystals (so called olivine- you add the terrestrial age to the cosmic exposure age phyric). Others have an abundance of both high- and (CRE) you get the time when the meteorite was low-Ca pyroxene, with poikilitic textures and were launched off of Mars by impact. Since only rather large originally called “lherzolitic shergottites”. But it has impacts would do this, there must be a finite number now been recognized that there is a more fundamental of such impacts – hence grouping of samples. and better way to describe these rocks (Irving 2012). Now that we have such a large number of samples, it Figure 1 shows a crude summary of CRE that have is time to understand what we have and infer what we been determined for Martian meteorites. Note that can about Mars. -
![(Sptpang Coil.) [I49] I50 Bulletin American Museum of Natural History](https://docslib.b-cdn.net/cover/0767/sptpang-coil-i49-i50-bulletin-american-museum-of-natural-history-200767.webp)
(Sptpang Coil.) [I49] I50 Bulletin American Museum of Natural History
Article VIII.-CATALOGUE OF METEORITES IN THE COLLECTION OF THE AMERICAN MUSEUM OF NATURAL HISTORY, TO JULY i, I896. By E. 0. HOVEY. 'T'he Collection of Meteorites in the Arnerican Museum of Natural History consists of fifty-five slabs, fragments and com- plete individuals, representing twenty-six falls and finds. The foundation of the mineralogical department of the Museum was laid in I874 by the purchase of the collection of S. C. H. Bailey, in which there were a few meteorites. More were acquired with the portion of the Norman Spang Collection of Minerals which was purchased in I89I, and other meteorites have been bought by the Museum from time to time, or have been presented to it by friends. The soujrce from which each specimen came has been indicated in the following cataloguLe. This publication is made to assist. the large number of persons who have become interested in knowing the extent to which the material of various falls and finds has been distributed among collections and the present location of specimens. AEROSIDERITES. (IRON METEORI ES.) Cat. Date of NAME AND Weight No. Discovery. DE;SCRIPTION. in grams. 18 1784 Tejupilco, Toluca Valley, Mexico. A complete individual, the surface of which has scaled off somewhat. A polished and etched surface shows coarse Widmanstatten figures. 1153. (Bailey Co/i.) 17841 Xiquipilco, Toluca Valley, Mexico. A complete individual of ellipsoidal form, which had been used as a pounder by the natives. 564. (Sptpang Coil.) [I49] I50 Bulletin American Museum of Natural History. [Vol. VIII, AEROSIDERITES.-Continued. Cat. Date of NAME AND DESCRIPTION. -

Iron Meteorites Are Made of Fe-Ni Metal Phases with Such Minor Minerals As Schrebersite, Troilite, Cohenite and Other Fe-Ni Carbides
Bulk elemental analyses of iron meteorites by using INAA and LA-ICPMS. N. Shirai1, A. Yamaguchi2, M. K. Haba2, T. Ojima2, M. Ebiahra1 and H. Kojima2, 1Tokyo Metropolitan Univer- sity, 2National Institute of Polar Research. Introduction: Iron meteorites are made of Fe-Ni metal phases with such minor minerals as schrebersite, troilite, cohenite and other Fe-Ni carbides. As most iron meteorites are believed to be samples from the metallic core of differentiated planetesimals, petrological, mineralogical and chemi- cal studies of iron meteorites are fundamental for unraveling the process of planetary differentiation. Based on the structures, iron meteorites are originally classified into hexahedrites, octahedrites and ataxites. Hexahedrites and ataxites are nearly made of kamacite and taenite, respectively. Octahedrites consist of kamacite and taenite, and they are further divided into six subgroups on the basis of the width of the kamcite from finest (>0.2 mm) to coarsest (>3.3 mm). Almost all iron meteorites are classified into octahedrites. The chemical clas- sification of iron meteorites is based on their trace element compositions (Ni, Ga, Ge and Ir). Bulk elemental abundances for iron meteorites have been obtained by using neutron activation analysis (NAA). Other analytical methods such as laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) have not been very often applied to iron meteorites. In this study, we present simple and effective procedures for the chemical classification of iron meteorites by using INAA and LA-ICPMS. Based on the analytical data obtained by two analytical techniques, we discuss the accuracy and the precision of our data and how promisingly our analytical methods can be applied to classifica- tion of iron meteorites.r 9, 2016 (12:00 pm, JST) Experimental: Canyon Diablo (IAB), Toluca (IAB), Cape York (IIIAB), Muonionalusta (IVA) and Dronino (ungrouped) were analyzed by using two analytical methods (INAA and LA-ICPMS). -

Lafayette - 800 Grams Nakhlite
Lafayette - 800 grams Nakhlite Figure 1. Photograph showing fine ablation features Figure 2. Photograph of bottom surface of Lafayette of fusion crust on Lafayette meteorite. Sample is meteorite. Photograph from Field Museum Natural shaped like a truncated cone. This is a view of the top History, Chicago, number 62918. of the cone. Sample is 4-5 centimeters across. Photo- graph from Field Museum Natural History, Chicago, number 62913. Introduction According to Graham et al. (1985), “a mass of about 800 grams was noticed by Farrington in 1931 in the geological collections in Purdue University in Lafayette Indiana.” It was first described by Nininger (1935) and Mason (1962). Lafayette is very similar to the Nakhla and Governador Valadares meteorites, but apparently distinct from them (Berkley et al. 1980). Lafayette is a single stone with a fusion crust showing Figure 3. Side view of Lafayette. Photograph from well-developed flow features from ablation in the Field Museum Natural History, Chicago, number Earth’s atmosphere (figures 1,2,3). The specimen is 62917. shaped like a rounded cone with a blunt bottom end. It was apparently oriented during entry into the Earth’s that the water released during stepwise heating of atmosphere. Note that the fine ablation features seen Lafayette was enriched in deuterium. The alteration on Lafayette have not been reported on any of the assemblages in Lafayette continue to be an active field Nakhla specimens. of research, because it has been shown that the alteration in Lafayette occurred on Mars. Karlsson et al. (1992) found that Lafayette contained the most extra-terrestrial water of any Martian Lafayette is 1.32 b.y. -

Thursday, August 11, 2016 EARLY SOLAR SYSTEM CHRONOLOGY I 8:30 A.M
79th Annual Meeting of the Meteoritical Society (2016) sess701.pdf Thursday, August 11, 2016 EARLY SOLAR SYSTEM CHRONOLOGY I 8:30 a.m. Room B Chairs: Gregory Brennecka Audrey Bouvier 8:30 a.m. Kruijer T. S. * Kleine T. Tungsten Isotope Dichotomy Among Iron Meteorite Parent Bodies: Implications for the Timescales of Accretion and Core Formation [#6449] We report new combined Pt and W isotope data for IC, IIC, IIF, IIIE, and IIIF iron meteorites, with the ultimate aim of better understanding the variable pre-exposure 182W/184W signatures observed among different iron meteorite groups. 8:45 a.m. Tissot F. L. H. * Dauphas N. Grove T. L. Heterogeneity in the 238U/235U Ratios of Angrites [#6104] We report the 238U/235U ratios of six angrites. We find that the angrite-parent body was heterogeneous with regards to U isotopes. We correct the Pb-Pb ages of angrites and test their concordance with ages derived from short-lived chronometers. 9:00 a.m. Brennecka G. A. * Amelin Y. Kleine T. Combined 238U/235U and Pb Isotopics of Planetary Core Material: The Absolute Age of the IVA Iron Muonionalusta [#6296] We report a measured 238U/235U for the IVA iron Muonionalusta. This measured value requires an age correction of ~7 Myr to the previously published Pb-Pb age. This has major implications for our understanding of planetary core formation and cooling. 9:15 a.m. Cartwright J. A. * Amelin Y. Koefoed P. Wadhwa M. U-Pb Age of the Ungrouped Achondrite NWA 8486 [#6231] We report the U-Pb age for ungrouped achondrite NWA 8486 (paired with NWA 7325). -

Sequestration of Martian CO2 by Mineral Carbonation
ARTICLE Received 11 Jun 2013 | Accepted 24 Sep 2013 | Published 22 Oct 2013 DOI: 10.1038/ncomms3662 OPEN Sequestration of Martian CO2 by mineral carbonation Tim Tomkinson1, Martin R. Lee2, Darren F. Mark1 & Caroline L. Smith3,4,5 Carbonation is the water-mediated replacement of silicate minerals, such as olivine, by carbonate, and is commonplace in the Earth’s crust. This reaction can remove significant quantities of CO2 from the atmosphere and store it over geological timescales. Here we present the first direct evidence for CO2 sequestration and storage on Mars by mineral carbonation. Electron beam imaging and analysis show that olivine and a plagioclase feldspar- rich mesostasis in the Lafayette meteorite have been replaced by carbonate. The susceptibility of olivine to replacement was enhanced by the presence of smectite veins along which CO2-rich fluids gained access to grain interiors. Lafayette was partially carbonated during the Amazonian, when liquid water was available intermittently and atmospheric CO2 concentrations were close to their present-day values. Earlier in Mars’ history, when the planet had a much thicker atmosphere and an active hydrosphere, carbonation is likely to have been an effective mechanism for sequestration of CO2. 1 Scottish Universities Environmental Research Centre, Rankine Avenue, Scottish Enterprise Technology Park, East Kilbride G75 0QF, UK. 2 School of Geographical and Earth Sciences, University of Glasgow, Gregory Building, Lilybank Gardens, Glasgow G12 8QQ, UK. 3 Department of Earth Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK. 4 ESA ESTEC, Keplerlaan 1, 200 AG Noordwijk, The Netherlands. 5 UK Space Agency, Atlas Building, Harwell Oxford, Didcot, Oxfordshire OX11 0QX, UK. -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

Constraints on the Water, Chlorine, and Fluorine Content of the Martian Mantle
Meteoritics & Planetary Science 1–13 (2016) doi: 10.1111/maps.12624 Constraints on the water, chlorine, and fluorine content of the Martian mantle 1* 2,3 4 Justin FILIBERTO , Juliane GROSS , and Francis M. MCCubbin 1Department of Geology, Southern Illinois University, 1259 Lincoln Dr, MC 4324, Carbondale, Illinois 62901, USA 2Department of Earth and Planetary Sciences, Rutgers University, 610 Taylor Road, Piscataway, New Jersey 08854, USA 3Department of Earth and Planetary Sciences, The American Museum of Natural History, New York, New York 10024, USA 4NASA Johnson Space Center, Mail Code XI2, 2101 NASA Parkway, Houston, Texas 77058, USA *Corresponding author. E-mail: fi[email protected] (Received 30 July 2015; revision accepted 22 January 2016) Abstract–Previous estimates of the volatile contents of Martian basalts, and hence their source regions, ranged from nearly volatile-free through estimates similar to those found in terrestrial subduction zones. Here, we use the bulk chemistry of Martian meteorites, along with Martian apatite and amphibole chemistry, to constrain the volatile contents of the Martian interior. Our estimates show that the volatile content of the source region for the Martian meteorites is similar to the terrestrial Mid-Ocean-Ridge Mantle source. Chlorine is enriched compared with the depleted terrestrial mantle but is similar to the terrestrial enriched source region; fluorine is similar to the terrestrial primitive mantle; and water is consistent with the terrestrial mantle. Our results show that Martian magmas were not volatile saturated; had water/chlorine and water/fluorine ratios ~0.4–18; and are most similar, in terms of volatiles, to terrestrial MORBs. Presumably, there are variations in volatile content in the Martian interior as suggested by apatite compositions, but more bulk chemical data, especially for fluorine and water, are required to investigate these variations.