Accessory Abducens Nucleus and Conditioned Eye Retraction/ Nictitating Membrane Extension in Rabbit'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience
CNs 5, 7, 9, 10 - Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience PHARYNGEAL ARCH SET, CNS 5, 7, 9, 10 • They are derived from the pharyngeal (aka branchial) arches • They have special motor and autonomic motor functions CRANIAL NERVES EXIT FROM THE BRAINSTEM CN 5, the trigeminal nerve exits the mid/lower pons.* CN 7, the facial nerve exits the pontomedullary junction.* CN 9, the glossopharyngeal nerve exits the lateral medulla.* CN 10, the vagus nerve exits the lateral medulla.* CRANIAL NERVE NUCLEI AT BRAINSTEM LEVELS Midbrain • The motor trigeminal nucleus of CN 5. Nerve Path: • The motor division of the trigeminal nerve passes laterally to enter cerebellopontine angle cistern. Pons • The facial nucleus of CN 7. • The superior salivatory nucleus of CN 7. Nerve Path: • CN 7 sweeps over the abducens nucleus as it exits the brainstem laterally in an internal genu, which generates a small bump in the floor of the fourth ventricle: the facial colliculus • Fibers emanate from the superior salivatory nucleus, as well. Medulla • The dorsal motor nucleus of the vagus, CN 10 • The inferior salivatory nucleus, CN 9 1 / 3 • The nucleus ambiguus, CNs 9 and 10. Nerve Paths: • CNs 9 and 10 exit the medulla laterally through the post-olivary sulcus to enter the cerebellomedullary cistern. THE TRIGEMINAL NERVE, CN 5  • The motor division of the trigeminal nerve innervates the muscles of mastication • It passes ventrolaterally through the cerebellopontine angle cistern and exits through foramen ovale as part of the mandibular division (CN 5[3]). Clinical Correlation - Trigeminal Neuropathy THE FACIAL NERVE, CN 7  • The facial nucleus innervates the muscles of facial expression • It spans from the lower pons to the pontomedullary junction. -
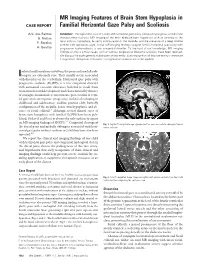
MR Imaging Features of Brain Stem Hypoplasia in Familial Horizontal
MR Imaging Features of Brain Stem Hypoplasia in CASE REPORT Familial Horizontal Gaze Palsy and Scoliosis A.V. dos Santos SUMMARY: We report the case of a child with horizontal gaze palsy, pendular nystagmus, and discrete S. Matias thoracolumbar scoliosis. MR imaging of the brain depicted pons hypoplasia with an absence of the facial colliculi, hypoplasia, butterfly configuration of the medulla, and the presence of a deep midline P. Saraiva pontine cleft (split pons sign). These MR imaging findings suggest familial horizontal gaze palsy with A. Goula˜o progressive kyphoscoliosis, a rare congenital disorder. To the best of our knowledge, MR imaging findings of only 4 similar cases, with or without progressive idiopathic scoliosis, have been reported. We discuss the pathogenesis substratum of this entity. Early recognition of this rare entity is important if supportive therapeutic measures in progressive scoliosis are to be applied. solated malformations involving the pons and medulla ob- Ilongata are extremely rare. They usually occur associated with disorders of the cerebellum. Horizontal gaze palsy with progressive scoliosis (HGPPS) is a rare congenital disorder with autosomal recessive inherence, believed to result from cranial nuclear maldevelopment and characterized by absence of conjugate horizontal eye movements, preservation of verti- cal gaze and convergence, progressive scoliosis developing in childhood and adolescence, midline pontine cleft, butterfly configuration of the medulla, brain stem hypoplasia, and ab- sence of facial colliculi.1 Although several clinical cases of brain stem hypoplasia with familial HGPPS have been pub- lished, Pieh et al and Rossi et al were the only authors to report on MR imaging findings of HGPPS.2,3 Congenital cleavage of Fig 1. -

Differentiation of the Bulbar Motor Nuclei and the Coincident Develop- Ment of Associated Root Fibers in the Rsbbit
DIFFERENTIATION OF THE BULBAR MOTOR NUCLEI AND THE COINCIDENT DEVELOP- MENT OF ASSOCIATED ROOT FIBERS IN THE RSBBIT DONALD L. KIMMEL Department of Anatomy, University of Michigan,’ Aim Arbor THIRTY-ONE FIGURES CONTENTS Introduction ........................................................ 83 Material and methods ................................................ 84 General survey of the literature ....................................... 85 Description of material studied ........................................ 86 Somatic efferent component. Its central and peripheral development .... 86 The hypoglossal ............................................. 86 The abdueens ............................................... 96 Visceral efferent component. Its central and peripheral development .... 103 The vago-accessory ........................................... 103 The glossopharyngeal ........................................ 124 The facial .................................................. 129 The trigemiiial .............................................. 137 Geiicrxldisrussion .................................................... 143 INTRODUCTION This present study concerns itself primarily with the onto- genetic development of the nuclear centers of bulbar cranial nerves in the rabbit and with the embryonic and adult distri- butions of their branches. Its purpose is to show that the central development proceeds stage for stage with the pro- A dissertation submitted in partial fulfillment of the requirements for the degree of doctor of philosophy -

Development and Developmental Disorders of the Brain Stem
Chapter 7 Development and Developmental Disorders of the Brain Stem Hans J.ten Donkelaar,Martin Lammens,Johannes R.M.Cruysberg and Cor W.J.R.Cremers 7.1 Introduction traocular muscles arise from mesomere 2 (the oculo- motor nucleus) and rhombomeres 1 (the trochlear The brain stem is composed of the midbrain (the nucleus) and 5 (the abducens nucleus). The motor mesencephalon) and the hindbrain (the rhomben- nuclei of the cranial nerves,innervating the branchial cephalon), and is, at least during development, seg- arch musculature,arise from the second,fourth,sixth mentally organized. The midbrain is composed of and seventh rhombomeres.The neural crest,flanking two temporarily present segments known as me- the developing rhombencephalon, makes important someres,whereas the hindbrain is composed of eight, contributions to the branchial arches (Chap. 5). A also temporarily present, rhombomeres (Fig. 7.1). great number of genes are involved in the proper de- The cerebellum largely arises from the first rhom- velopment of the brain stem (Cordes 2001; Moens bomere (Chap. 8). The brain stem contains the retic- and Prince 2002). The isthmus organizer regulates ular formation which is involved in the control of res- the early development of the mesencephalon and of piration, circulation, wakefulness and locomotion. the rostral part of the rhombencephalon (Wurst and The brain stem also contributes ten of the 12 pairs of Bally-Cuif 2001; Joyner 2002). Mutations of genes cranial nerves, III–XII. The motor nuclei for the ex- involved such as Otx2,En1 and En2 result in extensive Fig. 7.1 Segmentation of the brain stem (medial views of mus, is isthmus, Lc locus coeruleus, mes mesencephalon, the brain at Carnegie stages 12, 13, 15 and 17). -

Neuroanatomy, Cranial Nerve 6 (Abducens) - Statpearls - NCBI Bookshelf
12/17/2018 Neuroanatomy, Cranial Nerve 6 (Abducens) - StatPearls - NCBI Bookshelf NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Neuroanatomy, Cranial Nerve 6 (Abducens) Authors Van Nguyen; Matthew Varacallo1. Affilations 1 Department of Orthopaedic Surgery, University of Kentucky School of Medicine Last Update: October 27, 2018. Introduction Cranial nerve six (CN VI), also known as the abducens nerve, is one of the nerves responsible for the extraocular motor functions of the eye, along with the oculomotor nerve (CN III) and the trochlear nerve (CN IV). Structure and Function Unlike the oculomotor nerve and the trochlear nerve, the abducens nerve is a purely somatic nerve, meaning the nerve has no sensory function. Its main function is to carry general somatic efferent nerve axons to innervate the lateral rectus muscle which then abducts the eye on the ipsilateral side. It is also secondarily involved in innervation of the contralateral medial rectus muscle by way of the medial longitudinal fasciculus so that both eyes move laterally in a coordinated manner. Nerves The nerve itself can be divided into four distinct portions: the nucleus, the cisternal portion, the cavernous sinus portion, and the orbital portion. The abducens nucleus resides in the dorsal pons, ventral to the floor of the fourth ventricle, and just lateral to the medial longitudinal fasciculus. About forty percent of the axons project through the ipsilateral medial longitudinal fasciculus to cross over to the contralateral medial rectus subnucleus to eventually innervate the contralateral medial rectus muscle. -

Functional Architecture Underlying Binocular Coordination of Eye
Brysch et al. BMC Biology (2019) 17:110 https://doi.org/10.1186/s12915-019-0720-y RESEARCH ARTICLE Open Access Functional architecture underlying binocular coordination of eye position and velocity in the larval zebrafish hindbrain Christian Brysch1,2, Claire Leyden1,2 and Aristides B. Arrenberg1* Abstract Background: The oculomotor integrator (OI) in the vertebrate hindbrain transforms eye velocity input into persistent position coding output, which plays a crucial role in retinal image stability. For a mechanistic understanding of the integrator function and eye position control, knowledge about the tuning of the OI and other oculomotor nuclei is needed. Zebrafish are increasingly used to study integrator function and sensorimotor circuits, yet the precise neuronal tuning to motor variables remains uncharacterized. Results: Here, we recorded cellular calcium signals while evoking monocular and binocular optokinetic eye movements at different slow-phase eye velocities. Our analysis reveals the anatomical distributions of motoneurons and internuclear neurons in the nucleus abducens as well as those of oculomotor neurons in caudally adjacent hindbrain volumes. Each neuron is tuned to eye position and/or velocity to variable extents and is only activated after surpassing particular eye position and velocity thresholds. While the abducens (rhombomeres 5/6) mainly codes for eye position, in rhombomeres 7/8, a velocity-to-position coding gradient exists along the rostro-caudal axis, which likely corresponds to the oculomotor structures storing velocity and position, and is in agreement with a feedforward mechanism of persistent activity generation. Position encoding neurons are recruited at eye position thresholds distributed across the behaviourally relevant dynamic range, while velocity-encoding neurons have more centred firing thresholds for velocity. -

Brainstem II
Brainstem II Medical Neuroscience Dr. Wiegand Internal Brainstem | Cranial nerve nuclei | Location of selected tracts | Reticular formation Developmental Organization 1 Developmental Organization Sulcus Limitans Developmental Organization FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-14-1 2 Cranial Nerve Nuclei Organization | Medial to sulcus limitans z GSE ⇒ SVE ⇒ GVE | Lateral from sulcus limitans z VA ⇒ GSA ⇒ SSA FromFrom PritchardPritchard && Alloway:Alloway: Fig.Fig. 4-44-4 SEN MOT Generalizations | Sensory nuclei lateral to sulcus limitans | Motor nuclei medial to sulcus limitans | Visceral nuclei are on either side of sulcus | Innervation of skeletal muscle (GSE & SVE) most medial | General and special visceral afferent nuclei in same column I, II Cranial Nerves – Telencephalon & Diencephalon | Olfactory – z smell (SVA) | Optic – z vision (SSA) 3 III, IV Cranial Nerves – Mesencephalon | Oculomotor – z extraocular eye muscles (GSE) – oculomotor nucleus z PSNS to eye (GVE) – Edinger-Westphal nucleus | Trochlear – z extraocular muscle (sup. oblique) (GSE) – trochlear nucleus V, VI Cranial Nerves – Metencephalon | Trigeminal – z Masticatory muscles (SVE) – trigeminal motor nucleus z General sensation of the head and face (GSA) – trigeminal complex | Abducens – z extraocular muscle (lat. rectus) (GSE) – abducens nucleus VII Cranial Nerves – Metencephalon | Facial – z Facial expression muscles (SVE) – facial motor nucleus z Glands (submandibular, sublingual & lacrimal) (GVE) – superior salivatory & lacrimal nucleus z Taste (SVA) – rostral solitary nucleus z General sensation of ear (GSA) – trigeminal complex 4 VIII Cranial Nerves – Metencephalon Vestibulocochlear – z Hearing (SSA) – dorsal and ventral cochlear nuclei z Balance (SSA) – vestibular nuclei IX Cranial Nerves – Mylencephalon | Glossopharyngeal z Stylopharyngeus muscle (SVE) – n. ambiguus z PSNS to parotid gland (GVE) – inferior salivatory n. z Taste (SVA) – rostral solitary n. -

Lab 3. Pons & Midbrain
Lab 3. Pons & Midbrain Lesion Lessons Lesion 4.1 Anne T. Pasta i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; E.C. Alvord, Jr., Univ. of Washington) iii) Cause: Lesion 4.2 Colin S. Terase i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; M.Z. Jones, Michigan St. Univ.) iii) Cause: Medical Neuroscience 4– Pontine Level of the Facial Genu Locate and note the following: Basilar pons – massive ventral structure provides the most obvious change from previous med- ullary levels. Question classic • pontine gray - large nuclear groups in the basilar pons. Is the middle cerebellar peduncle composed – origin of the middle cerebellar peduncle of climbing or mossy • pontocerebellar axons - originate from pontine gray neurons and cross to form the fibers? middle cerebellar peduncle. • corticopontine axons- huge projection that terminates in the basilar pontine gray. • corticospinal tract axons – large bundles of axons surrounded by the basilar pontine gray. – course caudally to form the pyramids in the medulla. Pontine tegmentum • medial lemniscus - has now assumed a “horizontal” position and forms part of the border between the basilar pons and pontine tegmentum. Question classic • central tegmental tract - located just dorsally to the medial lemniscus. What sensory modali- – descends from the midbrain to the inferior olive. ties are carried by the • superior olivary nucleus - pale staining area lateral to the central tegmental tract. medial and lateral – gives rise to the efferent olivocochlear projection to the inner ear. lemnisci? • lateral lemniscus - lateral to the medial lemniscus. – composed of secondary auditory projections from the cochlear nuclei. -

Topography and Cytoarchitecture of the Motor Nuclei in the Brainstem of Salamanders
THE JOURNAL OF COMPARATIVE NEUROLOGY 278:181-194 (1988) Topography and Cytoarchitecture of the Motor Nuclei in the Brainstem of Salamanders GERHARD ROTH, RIISA NISHIKAWA, URSULA DICKE, AND DAVID B. WAKE Department of Biology, University of Bremen, 2800 Bremen 33, Federal Republic of Germany (G.R., U.D.); Museum of Vertebrate Zoology and Department of Zoology, University of California, Berkeley, California 94720 (K.N., D.B.W.) ABSTRACT The organization of the motor nuclei of cranial nerves V (including mesencephalic nucleus), VI, VII, IX, and X is described from HRP-stained material (whole mounts and sections) for 25 species representing five fami- lies of salamanders, and the general topology of the brainstem is considered. Location and organization of the motor nuclei, cytoarchitecture of each nucleus, and target organs for nuclei and subnuclei are described. The trigeminal nucleus is separated distinctly from the facial and abducens nuclei and consists of two subnuclei. The abducens nucleus consists of two distinct subnuclei, one medial in location, the abducens proper, and the other lateral, the abducens accessorius. The facial nucleus has two subnu- clei, and in all but one species it is posterior to the genu facialis. The facial nucleus completely overlaps the glossopharyngeal nucleus and partially overlaps that of the vagus. In bolitoglossine plethodontid salamanders, all of which have highly specialized projectile tongues, the glossopharyngeal and vagus nuclei have moved rostrally to overlap extensively and intermin- gle with the anterior and posterior subnuclei of the facial nerve. In the bolitoglossines there is less organization of the cells of the brainstem nuclei: dendritic trunks are less parallel and projection fields are wider than in other salamanders. -

A Review of Facial Nerve Anatomy
A Review of Facial Nerve Anatomy Terence M. Myckatyn, M.D.1 and Susan E. Mackinnon, M.D.1 ABSTRACT An intimate knowledge of facial nerve anatomy is critical to avoid its inadvertent injury during rhytidectomy, parotidectomy, maxillofacial fracture reduction, and almost any surgery of the head and neck. Injury to the frontal and marginal mandibular branches of the facial nerve in particular can lead to obvious clinical deficits, and areas where these nerves are particularly susceptible to injury have been designated danger zones by previous authors. Assessment of facial nerve function is not limited to its extratemporal anatomy, however, as many clinical deficits originate within its intratemporal and intracranial components. Similarly, the facial nerve cannot be considered an exclusively motor nerve given its contributions to taste, auricular sensation, sympathetic input to the middle meningeal artery, and parasympathetic innervation to the lacrimal, submandibular, and sublingual glands. The constellation of deficits resulting from facial nerve injury is correlated with its complex anatomy to help establish the level of injury, predict recovery, and guide surgical management. KEYWORDS: Extratemporal, intratemporal, facial nerve, frontal nerve, marginal mandibular nerve The anatomy of the facial nerve is among the components of the facial nerve reminds the surgeon that most complex of the cranial nerves. In his initial descrip- the facial nerve is composed not exclusively of voluntary tion of the cranial nerves, Galen described the facial motor fibers but also of parasympathetics to the lacrimal, nerve as part of a distinct facial-vestibulocochlear nerve submandibular, and sublingual glands; sensory innerva- complex.1,2 Although the anatomy of the other cranial tion to part of the external ear; and contributions to taste nerves was accurately described shortly after Galen’s at the anterior two thirds of the tongue. -
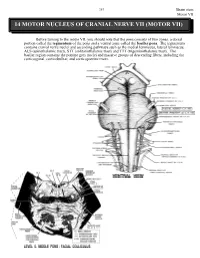
14 Motor Nucleus of Cranial Nerve Vii (Motor Vii)
263 Brain stem Motor VII 14 MOTOR NUCLEUS OF CRANIAL NERVE VII (MOTOR VII) Before turning to the motor VII, you should note that the pons consists of two zones, a dorsal portion called the tegmentum of the pons and a ventral zone called the basilar pons. The tegmentum contains cranial nerve nuclei and ascending pathways such as the medial lemniscus, lateral lemniscus, ALS (spinothalamic tract), STT (solitariothalamic tract) and TTT (trigeminothalamic tract). The basilar region contains the pontine grey nuclei and massive groups of descending fibers, including the corticospinal, corticobulbar, and corticopontine tracts. Brain stem 264 Motor VII The motor nucleus VII contains motor neurons (branchiomotor) that innervate the muscles of facial expression including the orbicularis oculi (CLOSES eyelid), the stapedius, the stylohyoid and the posterior belly of the digastric. Neurons comprising motor VII possess axons that pursue a rather circuitous route in order to exit the brain stem. Initially they pass dorsally and medially to loop over the abducens nucleus. The fibers then course ventrally and laterally to exit the brain stem. The bump in the floor of the fourth ventricle caused by the motor fibers of C.N. VII looping over the abducens nucleus is called the FACIAL COLLICULUS. A unilateral lesion interrupting the axons of C.N. VII results in the following: On the ipsilateral side, the forehead is immobile, the corner of the mouth sags, the nasolabial folds of the face are flattened, facial lines are lost, and saliva may drip from the corner of the mouth. The patient is unable to whistle or puff the cheek because the buccinator muscle is paralyzed. -

Identification of Functional Cell Groups in the Abducens Nucleus Of
ORIGINAL RESEARCH published: 19 June 2018 doi: 10.3389/fnana.2018.00045 Identification of Functional Cell Groups in the Abducens Nucleus of Monkey and Human by Perineuronal Nets and Choline Acetyltransferase Immunolabeling Anja K. E. Horn 1,2*, Annie Horng 3, Norbert Buresch 4, Ahmed Messoudi 1 and Wolfgang Härtig 5 1Anatomisches Institut, Ludwig-Maximilians Universität, München, Germany, 2Deutsches Schwindel- und Gleichgewichtszentrum, Ludwig-Maximilians Universität, München, Germany, 3RZM—Radiologisches Zentrum München-Pasing, München, Germany, 4Institut für Neuropathologie, Ludwig-Maximilians Universität, München, Germany, 5Paul-Flechsig-Institut für Hirnforschung, Universität Leipzig, Leipzig, Germany The abducens nucleus (nVI) contains several functional cell groups: motoneurons of the singly-innervated twitch muscle fibers (SIF) and those of the multiply-innervated muscle fibers (MIF) of the lateral rectus muscle (LR), internuclear neurons (INTs) projecting to the contralateral oculomotor nucleus (nIII) and paramedian tract-neurons (PMT) that receive input from premotor neurons of the oculomotor system and project to the floccular region. In monkey, these cell populations can be delineated by their chemical signature. For correlative clinico-pathological studies the identification of the homologous cell groups in the human nVI are required. In this study, we plotted the distribution of these populations in monkey nVI by combined tract-tracing and immunohistochemical Edited by: staining facilitating the identification of homologous cell