MMWR, Volume 70, Issue 28 — July 16, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advocacy Update Other News 2021 MIPS Exception Application Window Open Until Dec. 31
July 16, 2021: Advocacy Update other news 2021 MIPS exception application window open until Dec. 31 The COVID-19 pandemic has impacted all clinicians across the United States and territories. As a result, Centers for Medicare & Medicaid Services (CMS) continues to use its extreme and uncontrollable circumstances policy to allow clinicians, groups, virtual groups and Alternative Payment Model (APM) entities to submit an application requesting reweighting of 2021 Merit-Based Incentive Program (MIPS) performance categories due to the current COVID-19 public health emergency. Physicians who apply to have all MIPS categories reweighted will be held harmless from a penalty of up to -9%. Therefore, the AMA greatly encourages physicians to apply for the exception, whether reporting traditional MIPS or the APM Performance Pathway (APP). The application period is open until Dec. 31. Extreme and uncontrollable circumstances are defined as rare events entirely outside of your control and the control of the facility in which you practice, such as COVID-19. These circumstances would: Cause you to be unable to collect information necessary to submit for a MIPS performance category. Cause you to be unable to submit information that would be used to score a MIPS performance category for an extended period of time (for example, if you were unable to collect data for the quality performance category for 3 months). Impact your normal processes, affecting your performance on cost measures and other administrative claims measures. To register, you need a HCQIS Access Roles and Profile (HARP) account to submit an Extreme and Uncontrollable Circumstances Application. Once you sign into QPP with your HARP credentials, click "Exceptions Application” on the left-hand navigation and then select "Extreme and Uncontrollable Circumstances." Surgeon General advisory: Confronting health misinformation Surgeon General Vivek H. -

Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No
Pricing*, Pool and Payment** Due Dates January - December 2021 Mideast Marketing Area Federal Order No. 33 Class & Market Administrator Payment Dates for Producer Milk Component Final Pool Producer Advance Prices Payment Dates Final Payment Due Partial Payment Due Pool Month Prices Release Date Payrolls Due & Pricing Factors PSF, Admin., MS Cooperative Nonmember Cooperative Nonmember January February 3 * February 13 February 22 December 23, 2020 February 16 ** February 16 February 17 Janaury 25 January 26 February March 3 * March 13 March 22 January 21 * March 15 March 16 March 17 February 25 February 26 March March 31 * April 13 April 22 February 18 * April 15 April 16 April 19 ** March 25 March 26 April May 5 May 13 May 22 March 17 * May 17 ** May 17 ** May 17 April 26 ** April 26 May June 3 * June 13 June 22 April 21 * June 15 June 16 June 17 May 25 May 26 June June 30 * July 13 July 22 May 19 * July 15 July 16 July 19 ** June 25 June 28 ** July August 4 * August 13 August 22 June 23 August 16 ** August 16 August 17 July 26 ** July 26 August September 1 * September 13 September 22 July 21 * September 15 September 16 September 17 August 25 August 26 September September 29 * October 13 October 22 August 18 * October 15 October 18 ** October 18 ** September 27 ** September 27 ** October November 3 * November 13 November 22 September 22 * November 15 November 16 November 17 October 25 October 26 November December 1 * December 13 December 22 October 20 * December 15 December 16 December 17 November 26 ** November 26 December January 5, 2022 January 13, 2022 January 22, 2022 November 17 * January 18, 2022 ** January 18, 2022 ** January 18, 2022 ** December 27 ** December 27 ** * If the release date does not fall on the 5th (Class & Component Prices) or 23rd (Advance Prices & Pricing Factors), the most current release preceding will be used in the price calculation. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Case Management Continuance Chart (Pursuant to Law Div
CASE MANAGEMENT CONTINUANCE CHART (PURSUANT TO LAW DIV. GAO 20-4, SEC. 1.2) *MARCH 17 CONTINUED TO JULY 16 *MARCH 18 CONTINUED TO JULY 17 *MARCH 19 CONTINUED TO JULY 20 *MARCH 20 CONTINUED TO JULY 21 *MARCH 23 CONTINUED TO JULY 22 *MARCH 24 CONTINUED TO JULY 23 *MARCH 25 CONTINUED TO JULY 24 *MARCH 26 CONTINUED TO JULY 27 *MARCH 27 CONTINUED TO JULY 28 *The above cases were previously continued pursuant to Judge Evans General Administrative Order 20-01 that was entered March 13, 2020. The cases received new case management dates from April 16, 2020 to April 28, 2020. Therefore, the rescheduling of these cases use those April dates for calculating the new case management date. MARCH 30 CONTINUED TO JUNE 29 MARCH 31 CONTINUED TO JUNE 30 APRIL 01 CONTINUED TO JULY 01 APRIL 02 CONTINUED TO JULY 02 APRIL 03 CONTINUED TO JULY 06 APRIL 06 CONTINUED TO JULY 06 APRIL 07 CONTINUED TO JULY 07 APRIL 08 CONTINUED TO JULY 08 APRIL 09 CONTINUED TO JULY 09 APRIL 10 CONTINUED TO JULY 10 APRIL 13 CONTINUED TO JULY 13 APRIL 14 CONTINUED TO JULY 14 APRIL 15 CONTINUED TO JULY 15 APRIL 16 CONTINUED TO JULY 16 APRIL 17 CONTINUED TO JULY 17 APRIL 20 CONTINUED TO JULY 20 APRIL 21 CONTINUED TO JULY 21 APRIL 22 CONTINUED TO JULY 22 APRIL 23 CONTINUED TO JULY 23 APRIL 24 CONTINUED TO JULY 24 APRIL 27 CONTINUED TO JULY 27 APRIL 28 CONTINUED TO JULY 28 APRIL 29 CONTINUED TO JULY 29 APRIL 30 CONTINUED TO JULY 30 MAY 01 CONTINUED TO JULY 31 MAY 04 CONTINUED TO AUGUST 03 MAY 05 CONTINUED TO AUGUST 04 MAY 06 CONTINUED TO AUGUST 05 MAY 07 CONTINUED TO AUGUST 06 MAY 08 CONTINUED TO AUGUST 07 MAY 11 CONTINUED TO AUGUST 10 MAY 12 CONTINUED TO AUGUST 11 MAY 13 CONTINUED TO AUGUST 12 MAY 14 CONTINUED TO AUGUST 13 MAY 15 CONTINUED TO AUGUST 14 Please Note: Pursuant to Law Division General Administrative Order 20-4, all of the above cases, including those originally set for case management between March 17, 2020 and March 27, 2020, will have their case management date continued as detailed in the chart above. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
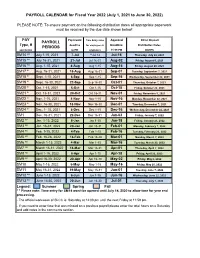
PAYROLL PERIODS PAYROLL CALENDAR for Fiscal Year 2022
PAYROLL CALENDAR for Fiscal Year 2022 (July 1, 2021 to June 30, 2022) PLEASE NOTE: To ensure payment on the following distribution dates all appropriate paperwork must be received by the due date shown below! PAY Paperwork Time Entry dates Approval Direct Deposit PAYROLL Type, # deadline for employees & Deadline Distribution Dates PERIODS see below to HR originators 11:59 PM DATES SM14 ª³ July 1-15, 2021 7-Jul **Jul 14 Jul-16 Thursday, July 22, 2021 SM15 ª³ July 16-31, 2021 21-Jul Jul 16-31 Aug-02 Friday, August 6, 2021 SM16 ª³ Aug. 1-15, 2021 4-Aug Aug 1-15 Aug-16 Friday, August 20, 2021 SM17 ª Aug. 16-31, 2021 18-Aug Aug 16-31 Sep-01 Tuesday, September 7, 2021 SM18 ª Sept. 1-15, 2021 8-Sep Sep 1-15 Sep-16 Wednesday, September 22, 2021 SM19 ª Sept. 16-30, 2021 22-Sep Sep 16-30 Oct-01 Thursday, October 7, 2021 SM20 ª Oct. 1-15, 2021 6-Oct Oct 1-15 Oct-18 Friday, October 22, 2021 SM21 ª Oct. 16-31, 2021 20-Oct Oct 16-31 Nov-01 Friday, November 5, 2021 SM22 ª Nov. 1-15, 2021 4-Nov Nov 1-15 Nov-16 Monday, November 22, 2021 SM23 ª Nov. 16-30, 2021 18-Nov Nov 16-30 Dec-01 Tuesday, December 7, 2021 SM24 ª Dec. 1- 15, 2021 8-Dec Dec 1-15 Dec-16 Wednesday, December 22, 2021 SM1 Dec. 16-31, 2021 22-Dec Dec 16-31 Jan-03 Friday, January 7, 2022 SM2 ª² Jan. -

Salary Payroll Schedule - 2021 Fiscal Pay Salary Overtime &Retro Leave Semester Pay Period HR Transaction Deadline Payday Days Year Number Entry (PHAHOUR) Periods
Salary Payroll Schedule - 2021 Fiscal Pay Salary Overtime &Retro Leave Semester Pay Period HR Transaction deadline Payday Days Year Number Entry (PHAHOUR) Periods December 25 – January 9 1 Monday, January 4, 2021 Wednesday, January 6, 2021 Friday, January 15, 2021 11 12 January 10 – January 24 2 Tuesday, January 19, 2021 Thursday, January 21, 2021 Monday, February 1, 2021 10 1 January 25 – February 9 3 Tuesday, February 2, 2021 Thursday, February 4, 2021 Tuesday, February 16, 2021 12 February 10 – February 24 4 Tuesday, February 16, 2021 Thursday, February 18, 2021 Monday, March 1, 2021 11 Spring Semester 2 Classes Begin February 25 – March 9 5 Wednesday, March 3, 2021 Friday, March 5, 2021 Tuesday, March 16, 2021 9 1/19/21 Exams End 5/13/21 21 March 10 – March 24 6 Thursday, March 18, 2021 Monday, March 22, 2021 Wednesday, March 31, 2021 11 3 March 25 – April 9 7 Monday, April 5, 2021 Wednesday, April 7, 2021 Friday, April 16, 2021 12 April 10 – April 24 8 Monday, April 19, 2021 Wednesday, April 21, 2021 Friday, April 30, 2021 10 4 April 25 – May 9 9 Monday, May 3, 2021 Wednesday, May 5, 2021 Friday, May 14, 2021 10 May 10 – May 24 10 Tuesday, May 18, 2021 Thursday, May 20, 2021 Tuesday, June 1, 2021 11 Summer I 5 Classes Begin May 25 – June 9 11 Thursday, June 3, 2021 Monday, June 7, 2021 Wednesday, June 16, 2021 12 5/24/21 (Paid 7/1/21) June 10 – June 24 12 Friday, June 18, 2021 Tuesday, June 22, 2021 Thursday, July 1, 2021 11 6 June 25 – July 9 13 Friday, July 2, 2021 Wednesday, July 7, 2021 Friday, July 16, 2021 11 Summer II Classes -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

July 16, 2020 Last Tuesday, Gov
July 16, 2020 Last Tuesday, Gov. Inslee announced he was going to stall counties from moving forward into the next phase of reopening until at least July 28 because of the current increase in positive cases throughout the state. He also cautioned that if cases continue to increase, some parts of the economy would have to take steps back and close again. During his press conference today, Gov. Inslee announced a rollback on social gatherings for counties currently in Phase 2 or modified Phase 1, reducing the number of people who could gather together to five and counties now in Phase 3 could gather in groups no larger than 10. The Governor indicated that if we do not adhere to mask wearing and social distancing, today’s rollbacks could be a forerunner of additional rollbacks. The increase in positive cases not just in Washington but nationally, has put greater emphasis on developing safe yet flexible plans for opening schools this fall. Yesterday’s case count increased by five positive cases to 49 in Jefferson County. Four of the five cases are members of the same family and are between the ages of 10 and 19, according to the Dept. of Public Health. “This is the time we have to get serious,” Locke said. If the transmission of the virus doesn’t slow down, it could impact the decision to open schools. “People have to take this seriously. The notion that a vaccine will be available and deployed by the end of the year is highly unlikely,” said Locke. In Jefferson County, facemasks are available at the following locations: Safeway Food Store, Sims Way, WA, 6am-10pm Food Coop, 414 Kearney Street, 8am-7pm Quilcene Village Store, Hwy. -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

The Final Rule
43304 Federal Register / Vol. 85, No. 137 / Thursday, July 16, 2020 / Rules and Regulations COUNCIL ON ENVIRONMENTAL B. Council on Environmental Quality E. Revisions to Commenting on QUALITY Regulations, Guidance, and Reports Environmental Impact Statements (Part 1. Regulatory History 1503) 40 CFR Parts 1500, 1501, 1502, 1503, 2. CEQ Guidance and Reports 1. Inviting Comments and Requesting 1504, 1505, 1506, 1507, 1508, 1515, 3. Environmental Impact Statement Information and Analyses (§ 1503.1) Timelines and Page Count Reports 2. Duty To Comment (§ 1503.2) 1516, 1517, and 1518 C. Judicial Review of Agency NEPA 3. Specificity of Comments and [CEQ–2019–0003] Compliance Information (§ 1503.3) D. Statutory Developments 4. Response to Comments (§ 1503.4) RIN 0331–AA03 E. Presidential Directives F. Revisions to Pre-Decisional Referrals to F. Advance Notice of Proposed Rulemaking the Council of Proposed Federal Actions Update to the Regulations G. Notice of Proposed Rulemaking Determined To Be Environmentally Implementing the Procedural II. Summary of Final Rule Unsatisfactory (Part 1504) A. Changes Throughout Parts 1500–1508 Provisions of the National 1. Purpose (§ 1504.1) B. Revisions To Update the Purpose, 2. Criterial for Referral (§ 1504.2) Environmental Policy Act Policy, and Mandate (Part 1500) 3. Procedure for Referrals and Response 1. Purpose and Policy (§ 1500.1) AGENCY: (§ 1504.3) Council on Environmental 2. Remove and Reserve Policy (§ 1500.2) Quality. G. Revisions to NEPA and Agency Decision 3. NEPA Compliance (§ 1500.3) Making (Part 1505) ACTION: Final rule. 4. Reducing Paperwork and Delay 1. Remove and Reserve Agency (§§ 1500.4 and 1500.5) Decisionmaking Procedures (§ 1505.1) SUMMARY: The Council on 5. -

July 16, 2021 for Immediate Release Counties of San Mateo, Alameda
County of San Mateo Joint Information Center Media Line: 650-779-9939 smc_ [email protected] July 16, 2021 For Immediate Release Counties of San Mateo, Alameda, Contra Costa, Marin, San Francisco, Santa Clara, Sonoma, and the City of Berkeley Recommend Masking Indoors for Everyone as a Precaution Against Increased Circulation of COVID-19 After falling in June, daily COVID-19 cases rising in San Mateo County With cases of COVID-19 rising locally and increased circulation of the highly transmissible Delta variant, the counties of San Mateo, Alameda, Contra Costa, Marin, San Francisco, Santa Clara, Sonoma, and the City of Berkeley recommend that everyone, regardless of vaccination status, wear masks indoors in public places to ensure easy verification that all unvaccinated people are masked in those settings and as an extra precautionary measure for all. In June, the Delta variants comprised 43 percent of all specimens sequenced in California. The Centers for Disease Control and Prevention (CDC) noted that Delta variants are now responsible for 58 percent of new infections across the country. Fully vaccinated people are well-protected from infections and serious illness due to known COVID-19 variants including Delta variants, and vaccinating as many people as possible, as soon as possible, continues to be our best defense against severe COVID-19 infection, and the harm it can do to our region. Vaccines are safe, effective, free, and widely available to everyone 12 and older. Out of an abundance of caution, people are recommended to wear masks indoors in settings like grocery or retail stores, theaters, and family entertainment centers, even if they are fully vaccinated as an added layer of protection for unvaccinated residents.