Phylum Platyhelminthes Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

(1104L) Animal Kingdom Part I
(1104L) Animal Kingdom Part I By: Jeffrey Mahr (1104L) Animal Kingdom Part I By: Jeffrey Mahr Online: < http://cnx.org/content/col12086/1.1/ > OpenStax-CNX This selection and arrangement of content as a collection is copyrighted by Jerey Mahr. It is licensed under the Creative Commons Attribution License 4.0 (http://creativecommons.org/licenses/by/4.0/). Collection structure revised: October 17, 2016 PDF generated: October 17, 2016 For copyright and attribution information for the modules contained in this collection, see p. 58. Table of Contents 1 (1104L) Animals introduction ....................................................................1 2 (1104L) Characteristics of Animals ..............................................................3 3 (1104L)The Evolutionary History of the Animal Kingdom ..................................11 4 (1104L) Phylum Porifera ........................................................................23 5 (1104L) Phylum Cnidaria .......................................................................31 6 (1104L) Phylum Rotifera & Phylum Platyhelminthes ........................................45 Glossary .............................................................................................53 Index ................................................................................................56 Attributions .........................................................................................58 iv Available for free at Connexions <http://cnx.org/content/col12086/1.1> Chapter 1 (1104L) Animals introduction1 -

Is the Turbellaria Polyphyletic?
Is the Turbellaria polyphyletic? Julian P. S. Smith, III,' Seth Teyler' & Reinhard M . Rieger2 'Department of Zoology, University of Maine, Orono, ME 04469, USA 2Department of Biology, University of North Carolina, Chapel Hill, NC 27514, USA Keywords: Tirbellaria, phylogenetic systematics, Platyhelminthes, polyphyly, ultrastructure, epidermis, cilia Abstract Within the last two decades, syntheses of both light-microscopic and ultrastructural characters have shown that there are three well-defined monophyletic groups within the Platyhelminthes : 1) the Catenulidale, 2) the Nemertodermatida-Acoela, and 3) the Haplopharyngida-Macrostomida-Polycladida-Neoophora (+ parasit- ic platyhelminth classes) . However, the relationships among these three groups are problematic . The possible apomorphies that would unite them are either not true homologues (i.e. frontal organ), are mutually conflict- ing (i.e. 9+1 axoneme in spermatozoa vs . biflagellate spermatozoa, epidermal ciliary rootlet structure, and protonephridia), or are unrooted with any outgroup and hence untestable or uncertain as apomorphies (pro- tonephridia, mode of epidermal replacement, absence of accessory centrioles on cilia) . The chief obstacle to deciphering the relationships of these groups is the lack of information on them ; presently available infor- mation is insufficient to test potential synapomorphies and insufficient also to allow agreement upon a nar- rowly defined outgroup for the Turbellaria . A view consistent with the present evidence (and admittedly an unsatisfactory -

Dear Author, Here Are the Proofs of Your Article. • You Can Submit Your
Dear Author, Here are the proofs of your article. • You can submit your corrections online, via e-mail or by fax. • For online submission please insert your corrections in the online correction form. Always indicate the line number to which the correction refers. • You can also insert your corrections in the proof PDF and email the annotated PDF. • For fax submission, please ensure that your corrections are clearly legible. Use a fine black pen and write the correction in the margin, not too close to the edge of the page. • Remember to note the journal title, article number, and your name when sending your response via e-mail or fax. • Check the metadata sheet to make sure that the header information, especially author names and the corresponding affiliations are correctly shown. • Check the questions that may have arisen during copy editing and insert your answers/ corrections. • Check that the text is complete and that all figures, tables and their legends are included. Also check the accuracy of special characters, equations, and electronic supplementary material if applicable. If necessary refer to the Edited manuscript. • The publication of inaccurate data such as dosages and units can have serious consequences. Please take particular care that all such details are correct. • Please do not make changes that involve only matters of style. We have generally introduced forms that follow the journal’s style. Substantial changes in content, e.g., new results, corrected values, title and authorship are not allowed without the approval of the responsible editor. In such a case, please contact the Editorial Office and return his/her consent together with the proof. -

II. Sampling Design
National Park Service U.S. Department of the Interior Natural Resource Program Center Protocol for Monitoring Aquatic Invertebrates of Small Streams in the Heartland Inventory & Monitoring Network Natural Resource Report NPS/HTLN/NRR—2008/042 A Heartland Network Monitoring Protocol protecting the habitat of our heritage i ON THE COVER Herbert Hoover birthplace cottage at Herbert Hoover NHS, prescribed fire at Tallgrass Prairie NPres, aquatic invertebrate monitoring at George Washington Carver NM, the Mississippi River at Effigy Mounds NM. ii Protocol for Monitoring Aquatic Invertebrates of Small Streams in the Heartland Inventory & Monitoring Network David E. Bowles, Michael H. Williams, Hope R. Dodd, Lloyd W. Morrison, Janice A. Hinsey, Catherine E. Ciak, Gareth A. Rowell, Michael D. DeBacker, and Jennifer L. Haack National Park Service Heartland I&M Network Wilson’s Creek National Battlefield 6424 West Farm Road 182 Republic, Missouri 65738 June 2008 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado i The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer- reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. Natural Resource Reports are the designated medium for disseminating high priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. -

Catenulida, Platyhelminthes) and Its Intracellular Symbionts
Zoomorphology (2011) 130:261–271 DOI 10.1007/s00435-011-0135-y ORIGINAL PAPER Microanatomy of the trophosome region of Paracatenula cf. polyhymnia (Catenulida, Platyhelminthes) and its intracellular symbionts Nikolaus Leisch • Ulrich Dirks • Harald R. Gruber-Vodicka • Markus Schmid • Wolfgang Sterrer • Jo¨rg A. Ott Received: 13 June 2011 / Revised: 11 August 2011 / Accepted: 13 August 2011 / Published online: 14 September 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Marine catenulid platyhelminths of the genus morphologically reduced and most likely not functional. Paracatenula lack mouth, pharynx and gut. They live in a Cells containing needle-like inclusions in the reference symbiosis with intracellular bacteria which are restricted to species Paracatenula polyhymnia Sterrer and Rieger, 1974 the body region posterior to the brain. The symbiont- were thought to be sperm, and the inclusions interpreted as housing cells (bacteriocytes) collectively form the tropho- the sperm nucleus. Our analysis of similar cells and their some tissue, which functionally replaces the digestive tract. inclusions by EDX and Raman microspectroscopy docu- It constitutes the largest part of the body and is the most ments an inorganic spicule consisting of a unique magne- important synapomorphy of this group. While some other sium–phosphate compound. Furthermore, we identify the features of the Paracatenula anatomy have already been neoblast stem cells located underneath the epidermis. analyzed, an in-depth analysis of the trophosome region Except for the modifications due to the symbiotic lifestyle was missing. Here, we identify and characterize the com- and the enigmatic spicule cells, the organization of Para- position of the trophosome and its surrounding tissue by catenula cf. -

Phylum Platyhelminthes
Author's personal copy Chapter 10 Phylum Platyhelminthes Carolina Noreña Departamento Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales (CSIC), Madrid, Spain Cristina Damborenea and Francisco Brusa División Zoología Invertebrados, Museo de La Plata, La Plata, Argentina Chapter Outline Introduction 181 Digestive Tract 192 General Systematic 181 Oral (Mouth Opening) 192 Phylogenetic Relationships 184 Intestine 193 Distribution and Diversity 184 Pharynx 193 Geographical Distribution 184 Osmoregulatory and Excretory Systems 194 Species Diversity and Abundance 186 Reproductive System and Development 194 General Biology 186 Reproductive Organs and Gametes 194 Body Wall, Epidermis, and Sensory Structures 186 Reproductive Types 196 External Epithelial, Basal Membrane, and Cell Development 196 Connections 186 General Ecology and Behavior 197 Cilia 187 Habitat Selection 197 Other Epidermal Structures 188 Food Web Role in the Ecosystem 197 Musculature 188 Ectosymbiosis 198 Parenchyma 188 Physiological Constraints 199 Organization and Structure of the Parenchyma 188 Collecting, Culturing, and Specimen Preparation 199 Cell Types and Musculature of the Parenchyma 189 Collecting 199 Functions of the Parenchyma 190 Culturing 200 Regeneration 190 Specimen Preparation 200 Neural System 191 Acknowledgment 200 Central Nervous System 191 References 200 Sensory Elements 192 INTRODUCTION by a peripheral syncytium with cytoplasmic elongations. Monogenea are normally ectoparasitic on aquatic verte- General Systematic brates, such as fishes, -
The Magnitude of Global Marine Species Diversity
Please cite this article in press as: Appeltans et al., The Magnitude of Global Marine Species Diversity, Current Biology (2012), http:// dx.doi.org/10.1016/j.cub.2012.09.036 Current Biology 22, 1–14, December 4, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.09.036 Article The Magnitude of Global Marine Species Diversity Ward Appeltans,1,2,96,* Shane T. Ahyong,3,4 Gary Anderson,5 8WorldFish Center, Los Ban˜ os, Laguna 4031, Philippines Martin V. Angel,6 Tom Artois,7 Nicolas Bailly,8 9ARTOO Marine Biology Consultants, Southampton Roger Bamber,9 Anthony Barber,10 Ilse Bartsch,11 SO14 5QY, UK Annalisa Berta,12 Magdalena Błazewicz-Paszkowycz,_ 13 10British Myriapod and Isopod Group, Ivybridge, Phil Bock,14 Geoff Boxshall,15 Christopher B. Boyko,16 Devon PL21 0BD, UK Simone Nunes Branda˜o,17,18 Rod A. Bray,15 11Research Institute and Natural History Museum, Niel L. Bruce,19,20 Stephen D. Cairns,21 Tin-Yam Chan,22 Senckenberg, Hamburg 22607, Germany Lanna Cheng,23 Allen G. Collins,24 Thomas Cribb,25 12Department of Biology, San Diego State University, Marco Curini-Galletti,26 Farid Dahdouh-Guebas,27,28 San Diego, CA 92182, USA Peter J.F. Davie,29 Michael N. Dawson,30 Olivier De Clerck,31 13Laboratory of Polar Biology and Oceanobiology, University Wim Decock,1 Sammy De Grave,32 Nicole J. de Voogd,33 of Ło´ dz, Ło´ dz 90-237, Poland Daryl P. Domning,34 Christian C. Emig,35 Christer Erse´us,36 14Museum Victoria, Melbourne, VIC 3000, Australia William Eschmeyer,37,38 Kristian Fauchald,21 15Department of Life Sciences, Natural History Museum, Daphne G. -

A Taxonomic Revision of South American Species of the Genus Stenostomum O
Zoological Journal of the Linnean Society, 2005, 144, 37-58. With 5 figures A taxonomic revision of South American species of the genus Stenostomum O. Schmidt (Platyhelminthes: Catenulida) based on morphological characters CAROLINA NOREÑA1*, CRISTINA DAMBORENEA2 and FRANCISCO BRUSA2 1CSIC, Departamento de Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales, Madrid, Spain 2CONICET, División Zoología Invertebrados, Facultad de Ciencias Naturales y Museo, UNLP, La Plata, Argentina Received May 2003; accepted for publication November 2004 This paper revises the genus Stenostomum based on a rich collection of species from Argentina and on a review of bibliographical resources of all known South American species. The description of South American species is stand ardized. The new genus Anokkostenostomum is established and several species of Stenostomum are transferred to it based on the absence of light-refracting bodies and the presence of a metameric anterior brain lobe. We report five Stenostomum species and three Anokkostenostomum species new to Argentina and present an identification key for South American species. Finally, all the world species are compiled, with an overview of their distribution. © 2005 The Linnean Society of London, Zoological Journal of the Linnean Society, 2005, 144, 37-58. ADDITIONAL KEYWORDS: genus revision - Neotropical region - species redescription - Stenostomum sp. - ‘Turbellaria’. INTRODUCTION Whereas Myostenostomum, Xenostenostomum and Rhynchoscolex contain few species and have promi Studies based on morphology (Ehlers, 1985, 1986; Ax, nent morphological characters, Stenostomum includes 1987) and on molecular data (Rohde et al., 1993; Cam approximately 50 species with a wide range of mor pos et al., 1998) confirm the Catenulida as a basal phological characteristics. -

28.3 Superphylum Lophotrochozoa – Flatworms, Rotifers, and Nemerteans
802 Chapter 28 | Invertebrates Figure 28.12 Hydrozoans. The polyp colony Obelia (a), siphonophore colonies Physalia (b) physalis, known as the Portuguese man o‘ war and Velella bae (c), and the solitary polyp Hydra (d) have different body shapes but all belong to the family Hydrozoa. (credit b: modification of work by NOAA; scale-bar data from Matt Russell) 28.3 | Superphylum Lophotrochozoa: Flatworms, Rotifers, and Nemerteans By the end of this section, you will be able to do the following: • Describe the unique anatomical and morphological features of flatworms, rotifers, and Nemertea • Identify an important extracoelomic cavity found in Nemertea • Explain the key features of Platyhelminthes and their importance as parasites Animals belonging to superphylum Lophotrochozoa are triploblastic (have three germ layers) and unlike the cnidarians, they possess an embryonic mesoderm sandwiched between the ectoderm and endoderm. These phyla are also bilaterally symmetrical, meaning that a longitudinal section will divide them into right and left sides that are superficially symmetrical. In these phyla, we also see the beginning of cephalization, the evolution of a concentration of nervous tissues and sensory organs in the head of the organism—exactly where a mobile This OpenStax book is available for free at http://cnx.org/content/col24361/1.8 Chapter 28 | Invertebrates 803 bilaterally symmetrical organism first encounters its environment. Lophotrochozoa are also protostomes, in which the blastopore, or the point of invagination of the ectoderm (outer germ layer), becomes the mouth opening into the alimentary canal. This developmental pattern is called protostomy or “first mouth.” Protostomes include acoelomate, pseudocoelomate, and eucoelomate phyla. -

An Introduction to the Invertebrates, Part Two Platyhelminthes & Rotifers
An Introduction to the Invertebrates, Part Two Platyhelminthes & Rotifers Reference: Chapter 33.3, 33.4 Quick Protist Review… v Are protists monophyletic, paraphyletic, or polyphyletic? v What are protozoa? v How do protists get energy? v How do protists reproduce? v Why are protists so diverse? v What are a few examples of protists? More Relationships The Bilaterians v Bilaterian animals have bilateral symmetry and triploblastic development (3 primary germ layers) v Most (but not all) have a coelom and a digestive tract with two openings v The clade Bilateria contains Acoelomorpha, Deuterostomia, Lophotrochozoa, and Ecdysozoa Phylum Acoelomorpha (Acoela in Fig. 32.11) v “Basal Bilaterians” § Bilateral symmetry § Acoelomate v Resemble flatworms (Phylum Platyhelminthes) § Simple nervous system and a saclike gut v Molecular evidence suggested Acoelomorphs diverged early from other bilaterians Superphylum Lophotrochozoa v The superphylum Lophotrochozoa was identified by molecular data v Some develop a lophophore for feeding, others pass through a trochophore larval stage, and a few have neither feature v Lophotrochozoa includes the flatworms, rotifers, ectoprocts, brachiopods, molluscs, and annelids Phylum Platyhelminthes – the Flatworms v Members of phylum Platyhelminthes live in marine, freshwater, and damp terrestrial habitats v Characteristics – they are FLAT! § Bilateral symmetry, triploblastic development, acoelomate § Flattened dorsoventrally § Gastrovascular cavity has one opening, the mouth § Gas exchange takes place by diffusion -
An Introduction to Invertebrates 667 � Figure 33.3 (Continued) Exploring Invertebrate Diversity
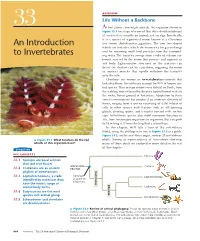
OVERVIEW Life Without a Backbone 33 At first glance, you might mistake the organism shown in Figure 33.1 for a type of seaweed. But this colorful inhabitant of coral reefs is actually an animal, not an alga. Specifically, it is a species of segmented worm known as a Christmas An Introduction tree worm (Spirobranchus giganteus). The two tree-shaped whorls are tentacles, which the worm uses for gas exchange and for removing small food particles from the surround- to Invertebrates ing water. The tentacles emerge from a tube of calcium car- bonate secreted by the worm that protects and supports its soft body. Light-sensitive structures on the tentacles can detect the shadow cast by a predator, triggering the worm to contract muscles that rapidly withdraw the tentacles into the tube. Christmas tree worms are invertebrates—animals that lack a backbone. Invertebrates account for 95% of known ani- mal species. They occupy almost every habitat on Earth, from the scalding water released by deep-sea hydrothermal vents to the rocky, frozen ground of Antarctica. Adaptation to these varied environments has produced an immense diversity of forms, ranging from a species consisting of a flat bilayer of cells to other species with features such as silk-spinning glands, pivoting spines, and tentacles covered with suction cups. Invertebrate species also show enormous variation in size, from microscopic organisms to organisms that can grow to 18 m long (1.5 times the length of a school bus). In this chapter, we’ll take a tour of the invertebrate world, using the phylogenetic tree in Figure 33.2 as a guide.