Patient Input
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cutaneous Manifestations of HIV Infection Carrie L
Chapter Title Cutaneous Manifestations of HIV Infection Carrie L. Kovarik, MD Addy Kekitiinwa, MB, ChB Heidi Schwarzwald, MD, MPH Objectives Table 1. Cutaneous manifestations of HIV 1. Review the most common cutaneous Cause Manifestations manifestations of human immunodeficiency Neoplasia Kaposi sarcoma virus (HIV) infection. Lymphoma 2. Describe the methods of diagnosis and treatment Squamous cell carcinoma for each cutaneous disease. Infectious Herpes zoster Herpes simplex virus infections Superficial fungal infections Key Points Angular cheilitis 1. Cutaneous lesions are often the first Chancroid manifestation of HIV noted by patients and Cryptococcus Histoplasmosis health professionals. Human papillomavirus (verruca vulgaris, 2. Cutaneous lesions occur frequently in both adults verruca plana, condyloma) and children infected with HIV. Impetigo 3. Diagnosis of several mucocutaneous diseases Lymphogranuloma venereum in the setting of HIV will allow appropriate Molluscum contagiosum treatment and prevention of complications. Syphilis Furunculosis 4. Prompt diagnosis and treatment of cutaneous Folliculitis manifestations can prevent complications and Pyomyositis improve quality of life for HIV-infected persons. Other Pruritic papular eruption Seborrheic dermatitis Overview Drug eruption Vasculitis Many people with human immunodeficiency virus Psoriasis (HIV) infection develop cutaneous lesions. The risk of Hyperpigmentation developing cutaneous manifestations increases with Photodermatitis disease progression. As immunosuppression increases, Atopic Dermatitis patients may develop multiple skin diseases at once, Hair changes atypical-appearing skin lesions, or diseases that are refractory to standard treatment. Skin conditions that have been associated with HIV infection are listed in Clinical staging is useful in the initial assessment of a Table 1. patient, at the time the patient enters into long-term HIV care, and for monitoring a patient’s disease progression. -

Chronic Paronychia Refers to a Skin Condition, Which Occurs Around the Nails
Robert E. Kalb, M.D. Buffalo Medical Group, P.C. Phone: (716) 630-1102 Fax: (716) 633-6507 Department of Dermatology 325 Essjay Road Williamsville, New York 14221 PARONYCHIA (CHRONIC) Chronic paronychia refers to a skin condition, which occurs around the nails. The term chronic means that the condition can come and go over time. The word paronychia is a fancy medical term referring to the inflammation, redness and swelling that can occur around the nails. Chronic paronychia occurs most commonly in people whose hands are in a wet environment, for example nurses, bartenders, dishwashers and hairdressers. Repeated cuts and minor trauma of the skin can damage the area around the nail and in the cuticle. This minor damage allows further irritation. There can be overgrowth of various surface germs, which slow the healing process. Symptoms of chronic paronychia include loss of the cuticle, tenderness, redness and swelling. Often the nails can appear changed with rough surfaces or grooves. Sometimes the area around the nail can be colonized with a normal bacteria or yeast on the skin. Because of this, one of the treatments that is often used is a medication, which has antibiotic properties against these types of organisms. In many cases, it is not an actual infection, but simply colonization on the surface of the skin, which impedes the healing. Treatment of chronic paronychia starts by avoiding any chronic irritation or wet environments. Wearing cotton-lined gloves to wash dishes can be helpful if this is an exposure. In most cases, topical medications are used. These often involve two different creams or two different liquids. -

Wrestling Skin Condition Report Form
IOWA HIGH SCHOOL ATHLETIC ASSOCIATION - WRESTLING SKIN CONDITION REPORT This is the only form a referee will accept as “current, written documentation” that a skin condition is NOT communicable. National Federation wrestling rules state, “If a participant is suspended by the referee or coach of having a communicable skin disease or any other condition that makes participation appear inadvisable, the coach shall provide current written documentation from an appropriate health-care professional, stating that the suspected disease or condition is not communicable and that the athlete’s participation would not be harmful to any opponent.” “COVERING A COMMUNICABLE CONDITION SHALL NOT BE CONSIDERED ACCEPTABLE AND DOES NOT MAKE THE WRESTLER ELIGIBLE TO PARTICIPATE.” This form must be presented to the referee, or opposing head coach, AT THE TIME OF WEIGH INS or the wrestler in question will not be allowed to compete. NFHS rule 4.2.5 states, “A contestant may have documentation from an appropriate health-care professional only, indicating a specific condi- tion such as a birthmark or other non-communicable skin conditions such as psoriasis and eczema, and that documentation is valid for the season. It is valid with the understanding that a chronic condition could become secondarily infected and may require re-evaluation. ________________________________________________ from ____________________________________ High School has Wrestler’s Name (Type or Print Legibly) High School Name (Type or Print Legibly) been examined by me for the following skin condition: ___________________________________________________________ Common name of skin condition here (Note: Wrestling coaches - the most common communicable wrestling skin conditions, and their medical names, are: boils - “furuncles” ; cold sores - “herpes simplex type-1”; impetigo - “pyoderma”; pink eye - “conjunctivitis”; ringworm - “tinea corporis”.) Mark the location(s) of the condition(s) on one of the sihlouettes below. -

Dermatologic Conditions Educational Format Faculty Expertise Required Expertise in the Field of Study
2019 AAFP FMX Needs Assessment Body System: Integumentary Session Topic: Dermatologic Conditions Educational Format Faculty Expertise Required Expertise in the field of study. Experience teaching in the field of study is desired. Preferred experience with audience Interactive REQUIRED response systems (ARS). Utilizing polling questions and Lecture engaging the learners in Q&A during the final 15 minutes of the session are required. Expertise teaching highly interactive, small group learning environments. Case-based, with experience developing and Problem- teaching case scenarios for simulation labs preferred. Other Based workshop-oriented designs may be accommodated. A typical OPTIONAL Learning PBL room is set for 50-100 participants, with 7-8 each per (PBL) round table. Please describe your interest and plan for teaching a PBL on your proposal form. Learning Objective(s) that will close Outcome Being Professional Practice Gap the gap and meet the need Measured Physicians have knowledge 1. Evaluate the presented skin condition Learners will gaps with regard to and determine differential diagnosis submit written diagnosing and evaluating and the need for further testing or commitment to common skin diseases (e.g. referral. change statements acne, dermatitis, rosacea). 2. Counsel patients on lifestyle on the session Primary care physicians modifications and proper skin care to evaluation, often receive inadequate control flare-ups and avoid outbreaks. indicating how dermatology training in 3. Create a disease management strategy they plan to medical school and for patients with a diagnosed implement residency. dermatologic condition based on the presented practice Patients with skin disease type and severity of the condition. recommendations. often have misconceptions 4. -

"Skin & Wound Management Under the Wraps"
SKIN & WOUND MANAGEMENT UNDER THE WRAPS Providing effective treatment and protection beneath compression bandaging is necessary to promote healing. Matthew Livingston, BSN, RN, CWS, ACHRN ound management for patients pustules around hair follicles, occurs and a drier skin surface dressing, such as living with venous insuffi- due to any type of trauma to the fol- cotton batting, will reduce the fungal ciency often involves multiple licle, such as pressure or friction, chemi- outbreak. Be aware that most rashes in W 1 complexities. These variations require cal irritation, or bacterial colonization. venous disease are from stasis dermatitis, providers to consider a spectrum of Milder forms of this skin condition are not candidiasis. strategies. Some require an advanced referred to as superficial folliculitis. This In its milder form, a latex allergy knowledge of skin conditions and dif- is considered self-limiting as long as the caused by compression wraps appears as ferential diagnosis while others are de- source of the injury is reduced. Painful, an itchy rash or hives over the major- pendent on “tricks of the trade” for deep folliculitis warrants a culture to ity of the lower extremity, or just above dressing changes and the understanding isolate the type of bacteria involved, and the knee (with possible systemic effects of different dressing modalities. treatment with systemic antibiotics.1 including puffy face and full-body rash). Fungal infections including candidia- The elastic component of the multilayer The ‘Skinny’ on Skin sis present as groups of small, red open compression dressing can be replaced Several dermatological conditions re- or closed pustules around the moist with a latex-free brand. -

Steatocystoma-Multiplex.Pdf
Steatocystoma multiplex Description Steatocystoma multiplex is a skin disorder characterized by the development of multiple noncancerous (benign) cysts known as steatocystomas. These growths begin in the skin's sebaceous glands, which normally produce an oily substance called sebum that lubricates the skin and hair. Steatocystomas are filled with sebum. In affected individuals, steatocystomas typically first appear during adolescence and are found most often on the torso, neck, upper arms, and upper legs. These cysts are usually the only sign of the condition. However, some affected individuals also have mild abnormalities involving the teeth or the fingernails and toenails. Frequency Although the prevalence of steatocystoma multiplex is unknown, it appears to be rare. Causes Steatocystoma multiplex can be caused by mutations in the KRT17 gene. This gene provides instructions for making a protein called keratin 17, which is produced in the nails, the hair follicles, and the skin on the palms of the hands and soles of the feet. It is also found in the skin's sebaceous glands. Keratin 17 partners with a similar protein called keratin 6b to form networks that provide strength and resilience to the skin, nails, and other tissues. The KRT17 gene mutations that cause steatocystoma multiplex alter the structure of keratin 17, preventing it from forming strong, stable networks within cells. The defective keratin network disrupts the growth and function of cells in the skin and nails, including cells that make up the sebaceous glands. These abnormalities lead to the growth of sebum-containing cysts in people with steatocystoma multiplex. However, it is unclear why steatocystomas are typically the only feature of this disorder. -

When Razor Meets Skin: a Scientific Approach to Shaving by Dr
When Razor Meets Skin: A Scientific Approach to Shaving by Dr. Diana Howard A survey conducted by The International Dermal Institute indicates that 79 percent of male respondents say they have one or more skin problem(s) that they notice daily, and yet the selection of their shaving products rarely takes this into account. Shaving can not only result in razor burn, ingrown hairs and razor bumps, but it can lead to increased sensitization and inflammation that results in premature aging. Unfortunately, as the average man’s beard grows two mm per day, there is ample opportunity to create an inflamed skin condition during shaving. As a matter of fact, if the average man starts shaving at age 13 and continues until he is 85 years old, and assuming he spends all of five minutes shaving each day, he will devote over six months of his life to just shaving his beard. As professional skin therapists, we may not be shaving our clients, but with the ever increasing number of men in skin treatment centers, salons and spas, we need to educate them about their specific skin care needs as it relates to shaving. Problems Associated with Shaving We know that the simple act of shaving imposes constant stress on the skin. Shaving is a form of physical exfoliation that can impact the health of the skin. Razor bumps, ingrown hairs, razor burn and inflammation are just some of the visible signs of trauma that the skin endures when a razor is used on the beard. Shaving triggers a high level of visible irritation and can lead to over- exfoliation, as well as a compromised lipid barrier. -
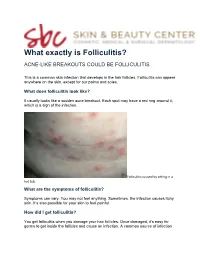
What Exactly Is Folliculitis?
What exactly is Folliculitis? ACNE-LIKE BREAKOUTS COULD BE FOLLICULITIS This is a common skin infection that develops in the hair follicles. Folliculitis can appear anywhere on the skin, except for our palms and soles. What does folliculitis look like? It usually looks like a sudden acne breakout. Each spot may have a red ring around it, which is a sign of the infection. Folliculitis caused by sitting in a hot tub. What are the symptoms of folliculitis? Symptoms can vary. You may not feel anything. Sometimes, the infection causes itchy skin. It’s also possible for your skin to feel painful. How did I get folliculitis? You get folliculitis when you damage your hair follicles. Once damaged, it’s easy for germs to get inside the follicles and cause an infection. A common source of infection is Staph aureus, which is found on our skin. Other organisms on our skin can also cause an infection. Also called pseudo folliculitis or razor bumps, men often see these on the beard area when they shave. You can damage your hair follicles by: • Touching or rubbing your skin frequently • Wearing tight clothing • Having skin rub against skin • Shaving When your skin is damp and hot, it’s easier to damage your hair follicles and get an infection. This can happen when tight clothing rubs against your skin while you’re bicycling on a hot day. The damage can also happen while you’re using a hot tub or whirlpool. When this occurs, the acne-like breakouts tend to appear on skin that was covered by your bathing suit. -

Pseudofolliculitis Barbae (Men)
Robert E. Kalb, M.D. Buffalo Medical Group, P.C. Phone: (716) 630-1102 Fax: (716) 633-6507 Department of Dermatology 325 Essjay Road Williamsville, New York 14221 PSEUDOFOLLICULITIS BARBAE (MEN) Pseudofolliculitis barbae is a long and confusing name, which applies to a skin condition, which affects the beard area of certain patients. This problem occurs because the hair tends to curl quickly and penetrates the skin. Where the hair penetrates the skin, a small red bump or pimple forms. Pseudofolliculitis is a problem, which is common in men. The reason why is that the hair tends to curl more quickly in some men. Since there is no way to change the way the hair grows, there is no true cure for this condition. Fortunately it can be controlled in the majority of cases. The main treatment for pseudofolliculitis barbae is to prevent the hair from curling back inward. A permanent solution is laser hair removal to destroy all the hair follicles. The problem with this approach is that it requires many sessions and is expensive. The other approaches include keeping the beard shaved very closely or by allowing a beard to grow. When a beard grows the hair is long enough that it cannot penetrate back in. Unfortunately for many patients growing a beard is not a reasonable alternative. The other option is to keep the beard shaved close so the hair never becomes long enough to penetrate the skin. There are many topical therapies, which help pseudofolliculitis barbae. One is topical Vaniqa cream which slows the growth of hair. -

Common Skin Conditions Explained Your Dermatology Pocket Guide: Common Skin Conditions Explained
Your Dermatology Pocket Guide: Common skin conditions explained Your Dermatology Pocket Guide: Common skin conditions explained Authors: Janice Bianchi Independent Medical Education Specialist Barbara Page, MBE Dermatology Liaison Nurse Specialist, NHS Fife Sheila Robertson Dermatology Liaison Nurse Specialist, NHS Fife © NES 2014 First Published June 2011 NESD0235 01 The structure and function of the skin 3 02 Taking a history 11 03 Examining the skin and describing lesions 17 Common skin conditions in adults 29 Acne 31 04 Cellulitis 35 Psoriasis 37 Shingles (herpes zoster) 40 Skin cancers 42 Vasculitis 49 Common skin conditions in children 51 Chicken pox (Varicella) 53 05 Eczema (atopic) 55 Henoch-Schönlein purpura 57 Impetigo 59 Impetiginised eczema 61 Miliaria 63 Measles 65 Molluscum Contagiosum 67 Napkin dermatitis (nappy rash) 69 Rubella (German measles) 71 Common skin conditions in both adults & children 73 Bites (insect) 75 06 Common Warts 77 Eczema herpeticum 79 Pruritus 81 Urticaria (acute) 83 Scabies 85 07 Practical advice on topical treatments 89 08 Glossary 97 09 Resources 105 Introduction 02 ////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////// This booklet can be used by any clinician who wishes to develop their knowledge of common skin conditions. It has been specifically written for nurse practitioners, specialist nurses, physicians or any other clinicians who undertake structured histories using advanced decision-making skills. It will aid in diagnosis - recognising differential diagnosis, formulating prescriptions and assisting in identifying referral pathways if necessary. The booklet is divided into three parts. Part 1 Part 1 (sections 01-03) starts with a review of the structure and functions of the skin, then takes the practitioner through history taking and describing the characteristics of the presenting skin condition. -

Dermatologist Cork | Alopecia Areata
Lee Clinic Dermatology Information Leaflet Alopecia Areata What is alopecia areata? Alopecia is a general term for hair loss. Alopecia areata is a specific, common cause of hair loss that can occur at any age. It usually causes small, coin- sized, round patches of baldness on the scalp, although hair elsewhere such as the beard, eyebrows, eyelashes, body and limbs can be affected. Occasionally it can involve the whole scalp (alopecia totalis) or even the entire body and scalp (alopecia universalis). It is not possible to predict how much hair will be lost. Regrowth of hair in typical alopecia areata is usual over a period of months or sometimes years, but cannot be guaranteed. The hair sometimes regrows white, at least in the first instance. Further hair loss is not uncommon. In alopecia totalis and alopecia universalis, the likelihood of total regrowth is less. What causes alopecia areata? Hair is lost because it is rejected by the affected person’s immune system, which does not recognise the hair roots (follicles) as "self", but regards them as "foreign" (autoimmunity). Why this happens is not fully understood, nor is it known why only localised areas are affected and why the hair regrows again. Someone with alopecia areata is more likely than a person without it to develop other autoimmune conditions such as thyroid disease, diabetes and vitiligo (white patches on the skin), although the risk of getting these disorders is still low. Your doctor may suggest a blood test looking for antibodies that may predict whether you are likely to develop thyroid problems or pernicious anaemia. -

WHAT YOU NEED to KNOW ABOUT SEYSARA® a Novel Treatment Developed Specifi Cally for Acne
Not an actual patient, results may vary. WHAT YOU NEED TO KNOW ABOUT SEYSARA® A novel treatment developed specifi cally for acne. PLEASE SEE THE ACCOMPANYING PATIENT INFORMATION AND FULL PRESCRIBING INFORMATION. almirall.us INTRODUCING SEYSARA: A NOVEL ORAL ANTIBIOTIC TREATMENT DESIGNED SPECIFICALLY FOR ACNE WHAT IS SEYSARA? WHAT CAUSES ACNE? SEYSARA is a prescription medicine used to treat moderate to Acne appears when a small hole in our skin (pore) clogs with dead severe acne vulgaris in people 9 years and older. SEYSARA should not skin cells. Normally, dead skin cells rise to the surface of the pore, be used for the treatment or prevention of infections. It is not known where they are shed. Excess production of sebum—the oil that keeps if SEYSARA is safe and effective for use for longer than 12 weeks. our skin from drying out—can cause the dead skin cells to stick SEYSARA should not be used in children under 9 years of age, or if you together and get trapped inside the pore. 1 are pregnant or breastfeeding. Sometimes the bacteria that live naturally on our skin, C. acnes, also get inside the pore, where they can multiply quickly. With WHAT IS MODERATE TO SEVERE ACNE? bacteria inside, the pore becomes infl amed (red and swollen). If the acne goes deep into the skin, an acne cyst or nodule appears.4 Acne is a common skin condition involving blockage and/or infl ammation of hair follicles and their associated gland. Depending on the severity, acne is generally categorized as mild, moderate, or severe.