INSTITUTE of MANAGEMENT and TECHNOLOGY (IMT) - THRISSUR (Owned by WESTFORT HIGHER EDUCATION TRUST) (Affiliated to University of Calicut and Approved by AICTE, Govt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accused Persons Arrested in Thrissur City District from 17.01.2021 to 23.01.2021
Accused Persons arrested in Thrissur City district from 17.01.2021 to 23.01.2021 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 MARUTHOOR KARTHIAYA Thrissur HOUSE, NI TEMPLE 23-01-2021 RAMACHA 33, 80/2021 U/s West BYJU K.C. SI BAILED BY 1 RAGHU KARTHIAYANI ROAD at 22:05 NDRAN Male 151 CrPC (Thrissur OF POLICE POLICE TEMPLE, AYYANTHO Hrs City) AYYANTHOLE LE Thrissur ODAYIL 23-01-2021 122/2021 U/s RADHAKRI 36, NR KALYAN East ANUDAS .K, BAILED BY 2 RAKESH (H),KUTTUMUKKU at 23:00 279 IPC & 185 SHNAN Male JEWELLERS (Thrissur SI OF POLICE POLICE , THRISSUR Hrs MV ACT City) Koothumakkal 23-01-2021 71/2021 U/s Peramangal 48, BAILED BY 3 Sreekumar Appu House, Varadiyam at 20:15 118(e) of KP am (Thrissur Sreejith S I Male POLICE Peringottukara Hrs Act City) CHOONDAKARA Thrissur N (H), NEAR 23-01-2021 26, 121/2021 U/s East ANUDAS .K, BAILED BY 4 THOMAS PAULSON THOTTAPPADY, SAPNA at 20:35 Male 279, 283 IPC (Thrissur SI OF POLICE POLICE ANCHERY, THEATRE Hrs City) THRISSUR ERATH (H), Thrissur 23-01-2021 119/2021 U/s 27, VALARKAVU, BTR ITC East ANUDAS .K, BAILED BY 5 SANOOP SUNIL at 19:00 279 IPC & 185 Male NAGAR, JUNCTION (Thrissur SI OF POLICE POLICE Hrs MV ACT KURIACHIRA City) KEEDAM KUNNATH(H)THI 51/2021 U/s PAZHAYA NIZAMUDDI 23-01-2021 GOPALAKR RAMAKRIS 39, RUVADI,PUDUKK PAZHAYAN 279 IPC & NNUR N J, BAILED BY 6 at -

The Heart of Kerala!
Welcome to the Heart of Kerala! http://www.neelambari.co.in w: +91 9400 525150 [email protected] f: http://www.facebook.com/NeelambariKerala Overview Neelambari is a luxurious resort on the banks of Karuvannur puzha (river). It is constructed in authentic Kerala style and evokes grandeur and tradition. The central building consists of a classical performance arena (Koothambalam) and a traditional courtyard (Nalukettu). The cottages are luxurious with their own private balconies, spacious and clean bathrooms and well appointed bedrooms (each unit has a space of more than 75 sqm). Neelambari is situated in a very serene atmosphere right on the bank of a river, in a quiet, verdant village in central Kerala. There are several natural and historical attractions in the vicinity. Despite its rural charm, the facility is well connected, being less than an hour drive from Cochin International Airport. It is also easily accessible by rail and road and the nearest city is Thrissur, just 13 kms away. The facility offers authentic Ayurveda treatment, Yoga lessons, nature and village tourism, kayak and traditional boat trips in the river as well as traditional cultural performances in its Koothambalam. http://www.neelambari.co.in w: +91 9400 525150 [email protected] f: http://www.facebook.com/NeelambariKerala Our location Neelambari is located in Arattupuzha, a serene little village in the outskirts of Thrissur City. Thrissur has a rightful claim as the cultural capital of Kerala for more reasons than one. A host of prestigious institutions that assiduously preserve and nurture the cultural traditions of Kerala such as the Kerala Sangeetha Nataka Academy, Kerala Sahitya Academy, Kerala Lalitha Kala Academy, Kerala Kalamandalam, Unnayi Warrier Kalanilayam are located in Thrissur. -
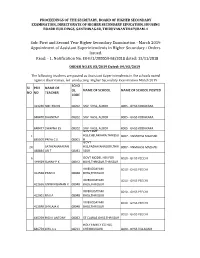
Sub: First and Second Year Higher Secondary Examination - March 2019 Appointment of Assistant Superintendents in Higher Secondary - Orders Issued
PROCEEDINGS OF THE SECRETARY, BOARD OF HIGHER SECONDARY EXAMINATION, DIRECTORATE OF HIGHER SECONDARY EDUCATION, HOUSING BOARD BUILDINGS, SANTHINAGAR, THIRUVANANTHAPURAM-1 Sub: First and Second Year Higher Secondary Examination - March 2019 Appointment of Assistant Superintendents in Higher Secondary - Orders Issued. Read: - 1. Notification No. EX-II/1/20000/HSE/2018 dated: 13/11/2018 ORDER NO.EX 03/2019 Dated: 04/03/2019 The following teachers are posted as Assistant Superintendents in the schools noted against their names, for conducting Higher Secondary Examination March 2019 SCHO SI PEN NAME OF OL NAME OF SCHOOL NAME OF SCHOOL POSTED NO NO TEACHER CODE 323246 SMITHA KN 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA 848470 DHANYA P 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA 848477 SWAPNA ES 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA GOVT SMT 1 HSS,CHELAKKARA,THRISSU 8007 - VNMGHSS MACHAD 835500 PRIYA C S 08001 R GOVT 24 SATHYANARAYAN HSS,PAZHAYANNOOR,THRI 8007 - VNMGHSS MACHAD 448844 AN T 08041 SSUR 6 GOVT MODEL HSS FOR 8010 - GHSS PEECHI 449429 SUNNY P K 08012 BOYS,THRISSUR,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412558 PRAN K 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412636 UNNIKRISHNAN K 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412601 RAJI A 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412598 SHYLAJA K 08048 BHSS,THRISSUR 8010 - GHSS PEECHI 645769 RIGI K ANTONY 08052 ST CLARAS GHSS,THRISSUR HOLY FAMILY CG HSS, 846759 LIRIL C L 08213 CHEMBUKAVU 8020 - GHSS VILLADAM SCHO SI PEN NAME OF OL NAME OF SCHOOL NAME OF SCHOOL POSTED NO NO TEACHER CODE ST AUGUSTINE HSS 846381 JINCY P FRANCIS 08215 KUTTANELLUR, THRISSUR 8020 - GHSS VILLADAM DENNIS XAVIER ST AUGUSTINE HSS 319862 AKKARA 08215 KUTTANELLUR, THRISSUR 8020 - GHSS VILLADAM 324459 SREEJISH P K 08206 CHENTHRA PINNI HSS 8022 - GHSS CHAVAKKAD ST. -

Ss of the Sector Product (S) Enterprise # 1 Haritham Arts&Crafts Traditional Handicraft Thrikaipetta (Po), Nellimalam, Wayanad T-9495240567
NAME & ADDRESS OF THE SECTOR PRODUCT (S) ENTERPRISE # 1 HARITHAM ARTS&CRAFTS TRADITIONAL HANDICRAFT THRIKAIPETTA (PO), NELLIMALAM, WAYANAD T-9495240567 2 ABHIRAMI BAMBOO UNIT TRADITIONAL BAMBOO PAZHUPPATHUR P.O PRODUCTS THEKKINKANDY WAYANAD-673592 T-9539595919 3 AISWARYA NUTIMIX FOOD NUTRIMIX NENMENIKKUNNU P.O PROCESSING SULTHAN BETHERY WAYANAD- 673595 T-9747075572 4 SWATHY CURRY POWDER FOOD CURRY POWDER THAVANI PROCESSING NENMENI P.O KOLIYADI SULTHAN BETHERY WAYANAD MOB: 9605974980 5 VALSALYAM NUTRIMIX FOOD CURRY POWDER/ NENMENI.P.O PROCESSING NUTRIMIX MADAKARA SULTHAN BETHERI WAYANAD MOB: 9656051316 [email protected] OM 6 AISWARYA NUTIMIX FOOD NUTRIMIX NOOLPUZHA PANJAYAT PROCESSING NENMENI KUNNU P.O WAYANAD- 673595 MOB: 9744540040 7 JWALA NUTRIMIX FOOD NUTRIMIX KIDAGANAD P O PROCESSING VADAKKANAD SULTHAN BETHERY WAYANAD MOB: 9961137711 8 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9744667166 9 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9747574461 10 ATHIRA FOOD PRODUCTS FOOD FOOD PRODUCT K21, USHAS PROCESSING KANIYARAM MANANTHAVADY WAYANAD PH: 04935240185 , 9446648051 11 AYSHA CHEMICALS CHEMICALS DETERGENTS POOTHICAUD POOMALA P O WAYANAD PH: 04936222166 12 KOCHIKUNNEL FOOD FOOD BAKERY PRODUCTS KINFRA PARK PROCESSING PRODUCTS CHUNDEL KALPETTA WAYANAD PH: 04936202707 , 9447042677 KOCHIKUNNELFOODPRODUCTS@G MAIL.COM 13 NAS BAGS OTHERS SHOPPER BAGS PUZHAMUDI P.O KALPETTA WAYANAD T-04936204669 , 9567455570 14 KENZ GARMENTS, GARMENTS READYMADE DWARAKA, -

1 TRICHUR 940 B Urban RBO 1 Thrissur 9654 2
ANNEXURE-A LIST OF BRANCHES UNDER RBO-I,THRISSUR BRANCH U/SU/RURAL Area(in SL. NO BRANCH NAME CODE CATEGORY REGION SQFT) 1 TRICHUR 940 B Urban RBO 1 Thrissur 9654 2 TRICHUR TOWN 8679 B Urban RBO 1 Thrissur 5438 3 PEECHI 2255 B Semi Urban RBO 1 Thrissur 2700 4 CHERPU 8606 B Rural RBO 1 Thrissur 2970 5 KURIACHIRA 8636 B Rural RBO 1 Thrissur 2595 6 VALLACHIRA 8684 B Semi Urban RBO 1 Thrissur 1670 7 VILANGAN 8693 B Rural RBO 1 Thrissur 3356 8 EAST FORT (TRICHUR) 9121 B Urban RBO 1 Thrissur 3356 Urban 9 POLICE ACADEMY TRICHUR 10566 B RBO 1 Thrissur 800 10 KUTTANELLUR 11930 B Urban RBO 1 Thrissur 2500 Urban RBO 1 Thrissur 11 SHAKTAN NAGAR TRICHUR 12892 B 1850 12 NRI TRICHUR 14466 B Urban RBO 1 Thrissur 1968 13 KUHAS TRICHUR 14682 B Rural RBO 1 Thrissur 3000 14 POONKUNNAM 16080 B Urban RBO 1 Thrissur 1995 15 SPBB THRISSUR 16085 B Urban RBO 1 Thrissur 2604 16 MANNUTHY 16494 B Urban RBO 1 Thrissur 1887 17 OLARI 16658 B Urban RBO 1 Thrissur 2998 18 PERINGAVU 18115 B Urban RBO 1 Thrissur 2947 19 KANJANI TOWN 18877 B Semi Urban RBO 1 Thrissur 1700 PUNKUNNAM RAILWAY Urban RBO 1 Thrissur 20 STATION, THRISSUR 21787 B 1650 21 THRISSUR- CIVIL STATION 70164 B Urban RBO 1 Thrissur 2165 22 THRISSUR- ROUND SOUTH 70165 B Urban RBO 1 Thrissur 3183 23 KURKANCHERRY 70174 B Urban RBO 1 Thrissur 2498 24 URAKOM 70175 B Semi Urban RBO 1 Thrissur 3300 25 CHERUR 70207 B Semi Urban RBO 1 Thrissur 3750 26 OLLUKARA 70210 B Urban RBO 1 Thrissur 4056 27 THRISSUR ADB 70253 B Semi Urban RBO 1 Thrissur 3400 28 OLLUR 70266 B Urban RBO 1 Thrissur 3416 29 THALORE 70470 B Semi -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Fri, 01 Oct 2021 00:50:50 GMT) CTRI Number CTRI/2017/10/009968 [Registered on: 03/10/2017] - Trial Registered Retrospectively Last Modified On 24/12/2018 Post Graduate Thesis No Type of Trial Observational Type of Study retrospective study Study Design Other Public Title of Study To evaluate safety and performance of the BioMime™- Sirolimus Eluting Coronary Stent System in the treatment of patients during a pre-defined period. Scientific Title of A Retrospective study of consecutive patients treated with BioMime™ – Sirolimus Eluting Coronary Study Stent System during a pre-defined period. Secondary IDs if Any Secondary ID Identifier NIL NIL Details of Principal Details of Principal Investigator Investigator or overall Name Dr Rajendra Kumar Premchand Jain Trial Coordinator (multi-center study) Designation Principal Investigator Affiliation Krishna Institute of Medical Sciences Address Department of Cardiology, Krishna Institute of Medical Sciences, 1-8-31/1, Minister Road, Begumpet, Hyderabad-500 003, Andhra Pradesh, India Hyderabad ANDHRA PRADESH 500 003 India Phone Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr Ashok Thakkar Query) Designation Head of Clinical Research Affiliation Meril Life Sciences Pvt Ltd Address Department of Clinical Resaerch, Meril Life Sciences Pvt Ltd, Bilakhia House, Survey No. 135/139, Muktanand Marg, Chala, Vapi Valsad GUJARAT 396191 India Phone 9879443584 Fax Email [email protected] Details Contact Details Contact Person (Public Query) Person (Public Query) Name Dr Ashok Thakkar Designation Head of Clinical Research Affiliation Meril Life Sciences PVT.LTD Address Meril Life Sciences Pvt. -
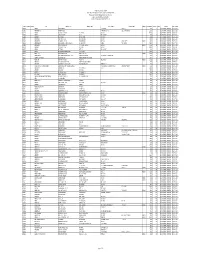
CSBL Unpaid Dividend, Refund Consolidated As on 22.09.2015.Xlsx
The Catholic Syrian Bank Limited Regd. Office, "CSB Bhavan", St. Mary's College Road, Thrissur 680020 Phone: 0487 -2333020, 6451640, eMail: [email protected] List of Unpaid Dividend as on 22.09.2015 (Dividend for the periods 2007-08 to 2013-14) FOLIO / DEMAT ID INITLS NAME ADDRESS LINE 1 ADDRESS LINE 2 ADDRESS LINE 3 ADDRESS LINE 4 PINCOD DIV.AMOUNT DWNO MICR PERIOD IEPF. TR. DATE A00350 ANTONY PALLANS HOUSE KURIACHARA TRICHUR, 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00385 ANNAMMA P X AKKARA HOUSE PANAMKUTTICHIRA OLLUR, TRICHUR DIST 150.00 5 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00398 ANTONY KUTTENCHERY HOUSE HIGH ROAD TRICHUR 1020.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00406 ANTONY KALLIATH HOUSE OLLUR TRICHUR DIST 27.00 9 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00409 ANTHONY PLOT NO 143 NEHRU NAGAR TRICHUR-6 120.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00643 ANTHAPPAN PADIKKALA HOUSE EAST FORT GATE TRICHUR 540.00 12 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00647 ANTHONY O K OLAKKENGAL HOUSE LOURDEPURAM TRICHUR - KERALA STATE. 680005 180.00 13 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00668 ANTHONISWAMI C/O INASIMUTHU MUDALIAR SONS 55 NEW STREET KARUR TAMILNADU 2100.00 14 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00822 ANNA JACOB C/O J S MANAVALAN 5 V R NAGAR ADAYAR MADRAS - 600020 210.00 18 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01072 ANTHONY VI/62 PALACE VIEW EAST FORT TRICHUR 4200.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01077 ANTONY KOTTEKAD KUTTUR TRICHUR DIST 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01103 ANTONY ELUVATHINGAL CHERUVATHERI -

Hospital List for Medicare Under Health Insurance| Royal Sundaram
SL.N STD O. HOSPITAL NAME ADDRESS - 1 ADDRESS - 2 CITY PIN CODE STATE ZONE CODE TEL 1 TEL - 2 FAX - 1 SALUTATION FIRST NAME MIDDLE SURNAME E MAIL ID (NEAR PEERA 1 SHRI JIYALAL HOSPITAL & MATERNITY CENTRE 6, INDER ENCLAVE, ROHTAK ROAD GARHI CHOWK) DELHI 110 087 DELHI NORTH 011 2525 2420 2525 8885 MISS MAHIMA 2 SUNDERLAL JAIN HOSPITAL ASHOK VIHAR, PHASE II DELHI 110 052 DELHI NORTH 011 4703 0900 4703 0910 MR DINESH K KHANDELWAL 3 TIRUPATI STONE CENTRE & HOSPITAL 6,GAGAN VIHAR,NEW DELHI DELHI 110051 DELHI NORTH 011 22461691 22047065 MS MEENU # 2, R.B.L.ISHER DAS SAWHNEY MARG, RAJPUR 4 TIRATH RAM SHAH HOSPITAL ROAD, DELHI 110054 DELHI NORTH 011 23972425 23953952 MR SURESH KUMAR 5 INDRAPRASTHA APOLLO HOSPITALS SARITA VIHAR DELHI MATHURA ROAD DELHI 110044 DELHI NORTH 011 26925804 26825700 MS KIRAN 6 SATYAM HOSPITAL A4/64-65, SECTOR-16, ROHINI, DELHI 110 085 DELHI NORTH 011 27850990 27295587 DR VIJAY KOHLI CS / OCF - 6 (NEAR POPULAR APARTMENT AND SECTOR - 13, 7 BHAGWATI HOSPITAL MOTHER DIARY BOOTH) ROHINI DELHI 110 085 DELHI NORTH 011 27554179 27554179 DR NARESH PAMNANI NETRAYATAN DR. GROVER'S CENTER FOR EYE 8 CARE S 371, GREATER KAILASH 2 DELHI 110 048 DELHI NORTH 011 29212828 29212828 DR VISHAL GROVER 9 SHROFF EYE CENTRE A-9, KAILASH COLONY DELHI 110048 DELHI NORTH 011 29231296 29231296 DR KOCHAR MADHUBAN 10 SAROJ HOSPITAL & HEART INSTITUTE SEC-14, EXTN-2, INSTITUTIONAL AREA CHOWK DELHI 110 085 DELHI NORTH 011 27557201 2756 6683 MR AJAY SHARMA 11 ADITYA VARMA MEDICAL CENTRE 32, CHITRA VIHAR DELHI 110 092 DELHI NORTH 011 2244 8008 22043839 22440108 MR SANOJ GUPTA SWARN CINEMA 12 SHRI RAMSINGH HOSPTIAL AND HEART INSTITUTE B-26-26A, EAST KRISHNA NAGAR ROAD DELHI 110 051 DELHI NORTH 011 209 6964 246 7228 MS ARCHANA GUPTA BALAJI MEDICAL & DIAGNOSTIC RESEARCH 13 CENTRE 108-A, I.P. -

Aided B.Ed Colleges Under Calicut University.Pdf
GO TO INDEX CATERGORY WISE LIST OF COLLEGES GO TO INDEX CATERGORY WISE LIST OF COLLEGES & INTAKE OF SEATS UPTO 02.11.2012 Sl.No Category Government Aided Unaided Total 2011-2012 2012-13 Total intake 1 Arts & Science 19 45 106 170 38895 1891 4 0786 2 Fine Arts 1 1 4 0 4 0 3 Engineering 3 1 32 36 13623 114 0 14 763 4 B.Arch 4 4 80 80 160 5 MBA/Management 8 8 660 60 720 6 Medical 2 5 7 1063 1063 7 Homeo 1 1 120 120 8 Ayurveda 2 4 6 312 312 9 Dental 1 6 7 376 376 10 Pharmacy 1 8 9 590 590 11 Paramedical Science 4 4 126 126 12 Nursing 2 19 21 1275 1275 13Law College 2 1 3 385 220 605 14 Physical Education 1 1 2 114 114 15 Training Colleges 2 2 59 63 7295 7295 16 Arabic Colleges 9 19 28 1066 180 124 6 Total 35 59 276 370 66020 3571 69591 GO TO INDEX INDEX I.THRISSUR 1.Arts & Science Colleges 1.1Government Colleges 1.2Aided Colleges 1.3Unaided Colleges 2.Fine Arts 2.1Government Colleges 3.Engineering Colleges 3.1Government Colleges 3.2Unaided Colleges 4.B.ARCH 4.1Unaided Colleges 5.MBA/Management Colleges 5.1Unaided Colleges 6.Medical Colleges 6.1Government Colleges 6.2Unaided Colleges 6.aDental 6.a.1Unaided Colleges 6.bAyurveda 6.b.1Aided Colleges 7.Pharmacy Colleges 7.1Unaided Colleges 8.Nursing Colleges 8.1Government Colleges 8.2Unaided Colleges 9.Law Colleges 9.1Government Colleges 10.Physical Education 10.1Unaided Colleges 11.Training Colleges GO TO INDEX 11.1Government Colleges 11.2Unaided Colleges 12.Arabic Colleges 12.1Unaided Colleges II.PALAKKAD 1. -

Download Prospectus
Self-Financing Paramedical Managements Association 2021-22 SELF FINANCING PARAMEDICAL MANAGEMENTS ASSOCIATION (SPMA) Allied health Streams Admission 2021-22 PROSPECTUS Admission office The Controller of Admission, SPMA Admission Office, MES Nursing College Campus, Palachode P.O, Kolathur Via, Malappuram, 679338. Ph.NO:04933-298418, Mob.no:9747315085, For technical support: 9846562040 Controller of Admissions- Mr.Sudheesh .K Administrative Office Regd.Office: Westfort Hospital Building PB No: 803, Thrissur-4, Phone: 0487-2382130, www.spma.in, Email:[email protected] 1 Self-Financing Paramedical Managements Association 2021-22 CONTENTS Clause Item Page Number 1 INTRODUCTION 03 01 Annex -A LIST OF PROGRAMME OFFERED 04 Annex-B DETAILS OF MEMBER INSTITUTION 05 02 2 CRITERIA OF ELIGIBILITY FOR ADMISSION 08 2.1 NATIONALITY 08 2.2 ACADEMIC 08 2.2.1 ADMISSION REQUIREMENTS 08 2.2.2 DURATION 09 2.3 RELAXATION IN MARKS 09 2.4 AGE 09 CENTRALIZED APPLICATION AND ALLOTMENT PROCESS 09 03 3 HOW TO APPLY 09 04 NRI QUOTA 11 4.1 HOW TO APPLY FOR NRI SEATS 11 05 SELECTION AND VERIFICATION OF DOCUMENTS 12 06 SPOT ALLOTMENT 12 07 GENERAL INFORMATION 13 08 MEDIUM OF INSTRUCTION 13 09 AFFILIATION OF COLLEGE 13 10 PROHIBITION OF RAGGING 13 11 SPECIAL INSTRUCTION 14 12 CODE OF CONDUCT 14 13 REFUND OF COURSE FEE/ LIQUIDATED DAMAGES 14 SCHEDULE OF ADMISSION PROCESS IS FIXED AS 14 15 FOLLOWS: 15 FEE STRUCTURE 16 16 NRI FEE 17 17 GRIEVANCE REDRESSAL 17 UNDERTAKING REGARDING NON-INVOLVEMENT IN 18 18 RAGGING 2 Self-Financing Paramedical Managements Association 2021-22 PREFACE The Self-Financing Paramedical Managements Association (SPMA) represents19- Paramedical college / institutes in Kerala (Registration no. -

Hymenoptera: Vespidae: Polistinae) from South India
Biological Forum Forum —– AnAn InternationalInternational Journal, Journal4,(2):1(1): 8-9(2012)12 -17 (2009) ISSN No. (Print) : 0975-1130 ISSN No. (Online) : 2249-3239 New Record of Polistes (Polistella) strigosus Bequaert (Hymenoptera: Vespidae: Polistinae) from South India Lambert Kishore, K.P. Mohammed Shareef and P. Girish Kumar* Malabar Christian College, Kozhikode, Kerala *Zoological Survey of India, New Alipore, Kolkata, (W.B.) (Received 23 , 2012, Accepted 25 May 2012) ABSTRACT : Polistes (Polistella) strigosus Bequaert is herewith recorded for the first time from South India and also from Western Ghats. Keywords : Polistes (Polistella) strigosus Bequaert, Western Ghats, Kerala, South India, new record. INTRODUCTION Das and Gupta and P. (P.) strigosus mimus Bequaert are recorded from Indian subcontinent of which P. (P.) strigosus The genus Polistes Latrielle is the cosmopolitan genus atratus Das and Gupta is reported from India (Assam, Bihar, which is most abundant and widely distributed among social Delhi, Manipur, Sikkim, Tripura, Uttarakhand and West Vespidae. They are commonly known as paper wasps and Bengal). None of them were reported from South India. The usually make relatively small colonies and usually build their black and brown colour pattern is highly variable in this nests in human inhabited areas. They are generally non- species. It requires further studies with more specimens aggressive compared to other social wasps but can be from different localities for confirming the status of different provoked into an aggressive morale for defending their colour variants (subspecies). So, at present, we are not nests. They are considered as beneficial insects since all dealing with the colour variants (subspecies) of this species the species are predatory and many consume large numbers here. -

COVID-19 Hospitals
Note: List updated on: 12-Jun-2021 1. This is a dynamic situation and facilities/resources listed are subject to change. Please call the labs/hospitals before visiting to make sure that they are providing the relevant services 2. Please check with the hospital administration before visiting the hospital about the bed availability S.No Hospital Name Address State City Pincode Konaseema Institute Of Medical 1 Nh 216 , Chaitanya Nagar Andhra Pradesh Amalapuram 533201 Science & Research Foundation H.No.28-1-56, Sangamesh Nagar,Opposite Indian Oil Petrol 2 SR Multispeciality Hospital Andhra Pradesh Anantapur 515001 Pump, Ananthapuram 3 Dr Ysr Memorial Hospitals 12-2-878, Sainagar 1St Cross, Near Apex Diagnostics Andhra Pradesh Anantapur 515001 15-11-154, Beside Of Vasavi Cloth Market, Mangalagiri 4 Vedanta Hospitals Andhra Pradesh Guntur 522001 Road D.NO 13-8-138, 8 th Lane, Near Guntur Bus Stand 5 Suraksha Hospitals( APJ Doctors LLP) Andhra Pradesh Guntur 522001 Gunturuvari Thota, Kothapelane Gunturuvarithota, 3Rd Line, Opp. Kamaraju Diagnostic 6 Aditya Multispeciality Hospital Andhra Pradesh Guntur 522001 Center 7 Samishta Hospital & Research Institute Kakumanu Vari Thota, 4th Line, Donka Road Andhra Pradesh Guntur 522002 8 Lalitha Super Speciality Hospital Pvt LtdKothapet ,Guntur Andhra Pradesh Guntur 522001 Guntur Kidney & Multi Speciality No. 15-11-1/10, Mangalagiri Road, Near Padmaja Petrol 9 Andhra Pradesh Guntur 522001 Hospital Bunk Amaravathi Institute Of Medical 10 Old Club Road, Kothapet Andhra Pradesh Guntur 522001 Sciences Pvt Ltd 11 Amrutha Hospitals Old Club Road, Kothapet Andhra Pradesh Guntur 522001 12 Kadapa Hospitals Christian Lane Opp:- Police Gate, City Union Bank Upstairs Andhra Pradesh Kadapa 516001 13 Mycure Hospital Site No.