Volume 24 Number 1 March 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Thesis Entitled Molecular, Morphological, and Biogeographic Resolution of Cryptic Taxa in the Greenside Darter Etheostoma Blen
A Thesis Entitled Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex By Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) ____________________________ Advisor: Dr. Carol A. Stepien ____________________________ Committee Member: Dr. Timothy G. Fisher ____________________________ Committee Member: Dr. Johan F. Gottgens ____________________________ College of Graduate Studies The University of Toledo December 2007 Copyright © 2007 This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Molecular, morphological, and biogeographic resolution of cryptic taxa in the Greenside Darter Etheostoma blennioides complex Amanda E. Haponski Submitted as partial fulfillment of the requirements for The Master of Science Degree in Biology (Ecology-track) The University of Toledo December 2007 DNA sequencing has led to the resolution of many cryptic taxa, which are especially prevalent in the North American darter fishes (Family Percidae). The Greenside Darter Etheostoma blennioides commonly occurs in the lower Great Lakes region, where two putative subspecies, the eastern “Allegheny” type E. b. blennioides and the western “Prairie” type E. b. pholidotum , overlap. The objective of this study was to test the systematic identity and genetic divergence distinguishing the two subspecies in areas of sympatry and allopatry in comparison to other subspecies and close relatives. DNA sequences from the mtDNA cytochrome b gene and control region and the nuclear S7 intron 1 comprising a total of 1,497 bp were compared from 294 individuals across 18 locations, including the Lake Erie basin and the Allegheny, Meramec, Obey, Ohio, Rockcastle, Susquehanna, and Wabash River systems. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Abstract ARCHAMBAULT, JENNIFER
Abstract ARCHAMBAULT, JENNIFER MICHELE. Thermal Sensitivity of Freshwater Mussels: Incorporating Benthic Ecology into Laboratory Mesocosm Experiments. (Under the direction of W. Gregory Cope and Thomas J. Kwak). The consequences of global climate change on aquatic ecosystems are predicted to result from altered intensity, variability, and distribution of precipitation, and more frequent flooding and droughts. In freshwater systems, these changes may result in degradation or loss of habitat due to dry stream beds or low flows, and increased water temperatures, pollution, and erosion. Freshwater mussels (Order Unionida) are especially vulnerable to disturbance because they are incapable of escaping detrimental changes at any practical temporal scale. Quantitative information on lethal temperatures (LT) to native freshwater mussels is currently limited to fewer than 10 species, and these few studies have been restricted to the water-only standard method for toxicity testing. The results of these prior studies indicate that some species may be living near their upper thermal tolerances; however, evaluation of the thermal sensitivity of these benthic organisms has never been conducted in sediment. Thus, I sought to increase the complexity and ecological realism of laboratory exposures of freshwater mussels to acute thermal stress by including factors that affect mussels in natural systems, including sediment, flow regime, and a vertical thermal gradient. I developed a method for assessing thermal sensitivity of freshwater mussels in sediment, and, using these testing protocols, I evaluated the relative sensitivities of juveniles of four species (Amblema plicata, Lampsilis abrupta, Lampsilis cariosa, and Lampsilis siliquoidea) and adults of one species (Lampsilis fasciola) to a range of temperatures common during summer in streams with low flow and drought conditions, using two temperature acclimation (22 and 27°C) and surrogate flow regimes (low water and dewatered treatments). -

1 Curriculum Vitae Stephen S. Curran, Ph.D. Department of Coastal
Curriculum vitae Stephen S. Curran, Ph.D. Department of Coastal Sciences The University of Southern Mississippi Gulf Coast Research Laboratory 703 East Beach Drive Phone: (228) 238-0208 Ocean Springs, MS 39564 Email: [email protected] Research and Teaching Interests: I am an organismal biologist interested in the biodiversity of metazoan parasitic animals. I study their taxonomy using traditional microscopic and histological techniques and their genetic interrelationships and systematics using ribosomal DNA sequences. I also investigate the effects of extrinsic factors on aquatic environments by using parasite prevalence and abundance as a proxy for total biodiversity in aquatic communities and for assessing food web dynamics. I am also interested in the epidemiology of viral diseases of crustaceans. University Teaching Experience: •Instructor for Parasites of Marine Animals Summer class, University of Southern Mississippi, Gulf Coast Research Laboratory (2011-present). •Co-Instructor (with Richard Heard) for Marine Invertebrate Zoology, University of Southern Mississippi, Gulf Coast Research Laboratory (2007). •Intern Mentor, Gulf Coast Research Laboratory. I’ve instructed 16 interns during (2003, 2007- present). •Graduate Teaching Assistant for Animal Parasitology, Department of Ecology and Evolutionary Biology, University of Connecticut (Spring 1995). •Graduate Teaching Assistant for Introductory Biology for Majors, Department of Ecology and Evolutionary Biology, University of Connecticut (Fall 1994). Positions: •Assistant Research -
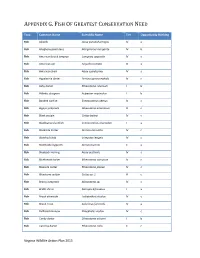
Fish of Greatest Conservation Need
APPENDIX G. FISH OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Fish Alewife Alosa pseudoharengus IV a Fish Allegheny pearl dace Margariscus margarita IV b Fish American brook lamprey Lampetra appendix IV c Fish American eel Anguilla rostrata III a Fish American shad Alosa sapidissima IV a Fish Appalachia darter Percina gymnocephala IV c Fish Ashy darter Etheostoma cinereum I b Fish Atlantic sturgeon Acipenser oxyrinchus I b Fish Banded sunfish Enneacanthus obesus IV c Fish Bigeye jumprock Moxostoma ariommum III c Fish Black sculpin Cottus baileyi IV c Fish Blackbanded sunfish Enneacanthus chaetodon I a Fish Blackside darter Percina maculata IV c Fish Blotched chub Erimystax insignis IV c Fish Blotchside logperch Percina burtoni II a Fish Blueback Herring Alosa aestivalis IV a Fish Bluebreast darter Etheostoma camurum IV c Fish Blueside darter Etheostoma jessiae IV c Fish Bluestone sculpin Cottus sp. 1 III c Fish Brassy Jumprock Moxostoma sp. IV c Fish Bridle shiner Notropis bifrenatus I a Fish Brook silverside Labidesthes sicculus IV c Fish Brook Trout Salvelinus fontinalis IV a Fish Bullhead minnow Pimephales vigilax IV c Fish Candy darter Etheostoma osburni I b Fish Carolina darter Etheostoma collis II c Virginia Wildlife Action Plan 2015 APPENDIX G. FISH OF GREATEST CONSERVATION NEED Fish Carolina fantail darter Etheostoma brevispinum IV c Fish Channel darter Percina copelandi III c Fish Clinch dace Chrosomus sp. cf. saylori I a Fish Clinch sculpin Cottus sp. 4 III c Fish Dusky darter Percina sciera IV c Fish Duskytail darter Etheostoma percnurum I a Fish Emerald shiner Notropis atherinoides IV c Fish Fatlips minnow Phenacobius crassilabrum II c Fish Freshwater drum Aplodinotus grunniens III c Fish Golden Darter Etheostoma denoncourti II b Fish Greenfin darter Etheostoma chlorobranchium I b Fish Highback chub Hybopsis hypsinotus IV c Fish Highfin Shiner Notropis altipinnis IV c Fish Holston sculpin Cottus sp. -
![Binder 021, Bucephalidae [Trematoda Taxon Notebooks]](https://docslib.b-cdn.net/cover/6980/binder-021-bucephalidae-trematoda-taxon-notebooks-316980.webp)
Binder 021, Bucephalidae [Trematoda Taxon Notebooks]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Trematoda Taxon Notebooks Parasitology, Harold W. Manter Laboratory of February 2021 Binder 021, Bucephalidae [Trematoda Taxon Notebooks] Harold W. Manter Laboratory of Parasitology Follow this and additional works at: https://digitalcommons.unl.edu/trematoda Part of the Biodiversity Commons, Parasitic Diseases Commons, and the Parasitology Commons Harold W. Manter Laboratory of Parasitology, "Binder 021, Bucephalidae [Trematoda Taxon Notebooks]" (2021). Trematoda Taxon Notebooks. 21. https://digitalcommons.unl.edu/trematoda/21 This Portfolio is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Trematoda Taxon Notebooks by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Family BUCEPHALIDAE POCHE, 1907 1. Bucephalus varicus Manter, 1940 Host. Caranx hippos (Linn.): Common jack; family Carangidae Incidence of Infection. In 1 of 1 host Location. Mainly close to pyloric junction and a few scattered speci mens along length of entire intestine Locality. Bayboro Harbor, Tampa Bay, (new locality record) Florida Discussion. Manter (1940) pictured variation of tentacles and displa- ---- -- cement of organs in preserved B. varicus from the Tropical American Pacific and Tortugas, Florida. We have studied live B. varicus under slight coverslip pressure and can confirm the variations observed by Manter ( 1940) . B. varicus has been reported from no less than eleven different carangid species from Okinawa, the Red Sea, Tortugas, Florida, and the Tropical American Pacific. The only other record of B. varicus from Caranx hippos is by Bravo and Sogandares (1957) from the Pacific Coast of Mexico. -

IN the KIAMICHI RIVER, OKLAHOMA PROJECTTITLE: Habitat Use and Reproductive Biology of Arkansia Whee/Eri (Mollusca: Unionidae) in the Kiamichi River, Oklahoma
W 2800.7 E56s No.E-12 1990/93 c.3 OKLAHOMA o HABITAT USE AND REPRODUCTIVE BIOLOGY OF ARKANSIA WHEELERI (MOLLUSCA: UNIONIDAE) IN THE KIAMICHI RIVER, OKLAHOMA PROJECTTITLE: Habitat use and reproductive biology of Arkansia whee/eri (Mollusca: Unionidae) in the Kiamichi River, Oklahoma. whee/eri is associated. In its optimal habitat, A. whee/eri is always rare: mean relative 2 abundance varies from 0.2 to 0.7% and the average density is 0.27 individuals/m • In addition, shell length data for live Amb/ema plicata, a dominant mussel species in the Kiamichi River, indicate reduced recruitment below Sardis Reservoir. Much of the Kiamichi River watershed remains forested and this probably accounts for the high diversity and general health of its mussel community in comparison to other nearby rivers. Program Narrative Objective To determine the distribution, abundance and reproductive biology of the freshwater mussel Arkansia whee/eri within different habitats in the Kiamichi River of Oklahoma. Job Procedures 1. Characterize microhabitats and determine the effects of impoundment. 2. Determine movement, growth, and survivorship of individuals. 3. Identify glochidia and fish host. 4. Examine impact of Sardis Reservoir on the populations. 5. Determine historic and current land use within the current range of Arkansia whee/eri in the Kiamichi River. A. Introduction Arkansia (syn. Arcidens) whee/eri, the Ouachita rock pocketbook, is a freshwater mussel. Originally named Arkansia whee/eri by Ortmann and Walker in 1912, Clarke (1981, 1985) recognized Arkansia as a subgenus of Arcidens. The species is considered by Clarke to be distinct. However, Turgeon et al (1988) have continued to use the binomial Arkansia whee/eri. -

Malaco Le Journal Électronique De La Malacologie Continentale Française
MalaCo Le journal électronique de la malacologie continentale française www.journal-malaco.fr MalaCo (ISSN 1778-3941) est un journal électronique gratuit, annuel ou bisannuel pour la promotion et la connaissance des mollusques continentaux de la faune de France. Equipe éditoriale Jean-Michel BICHAIN / Paris / [email protected] Xavier CUCHERAT / Audinghen / [email protected] Benoît FONTAINE / Paris / [email protected] Olivier GARGOMINY / Paris / [email protected] Vincent PRIE / Montpellier / [email protected] Les manuscrits sont à envoyer à : Journal MalaCo Muséum national d’Histoire naturelle Equipe de Malacologie Case Postale 051 55, rue Buffon 75005 Paris Ou par Email à [email protected] MalaCo est téléchargeable gratuitement sur le site : http://www.journal-malaco.fr MalaCo (ISSN 1778-3941) est une publication de l’association Caracol Association Caracol Route de Lodève 34700 Saint-Etienne-de-Gourgas JO Association n° 0034 DE 2003 Déclaration en date du 17 juillet 2003 sous le n° 2569 Journal électronique de la malacologie continentale française MalaCo Septembre 2006 ▪ numéro 3 Au total, 119 espèces et sous-espèces de mollusques, dont quatre strictement endémiques, sont recensées dans les différents habitats du Parc naturel du Mercantour (photos Olivier Gargominy, se reporter aux figures 5, 10 et 17 de l’article d’O. Gargominy & Th. Ripken). Sommaire Page 100 Éditorial Page 101 Actualités Page 102 Librairie Page 103 Brèves & News ▪ Endémisme et extinctions : systématique des Endodontidae (Mollusca, Pulmonata) de Rurutu (Iles Australes, Polynésie française) Gabrielle ZIMMERMANN ▪ The first annual meeting of Task-Force-Limax, Bünder Naturmuseum, Chur, Switzerland, 8-10 September, 2006: presentation, outcomes and abstracts Isabel HYMAN ▪ Collecting and transporting living slugs (Pulmonata: Limacidae) Isabel HYMAN ▪ A List of type specimens of land and freshwater molluscs from France present in the national molluscs collection of the Hebrew University of Jerusalem Henk K. -

December 2011
Ellipsaria Vol. 13 - No. 4 December 2011 Newsletter of the Freshwater Mollusk Conservation Society Volume 13 – Number 4 December 2011 FMCS 2012 WORKSHOP: Incorporating Environmental Flows, 2012 Workshop 1 Climate Change, and Ecosystem Services into Freshwater Mussel Society News 2 Conservation and Management April 19 & 20, 2012 Holiday Inn- Athens, Georgia Announcements 5 The FMCS 2012 Workshop will be held on April 19 and 20, 2012, at the Holiday Inn, 197 E. Broad Street, in Athens, Georgia, USA. The topic of the workshop is Recent “Incorporating Environmental Flows, Climate Change, and Publications 8 Ecosystem Services into Freshwater Mussel Conservation and Management”. Morning and afternoon sessions on Thursday will address science, policy, and legal issues Upcoming related to establishing and maintaining environmental flow recommendations for mussels. The session on Friday Meetings 8 morning will consider how to incorporate climate change into freshwater mussel conservation; talks will range from an overview of national and regional activities to local case Contributed studies. The Friday afternoon session will cover the Articles 9 emerging science of “Ecosystem Services” and how this can be used in estimating the value of mussel conservation. There will be a combined student poster FMCS Officers 47 session and social on Thursday evening. A block of rooms will be available at the Holiday Inn, Athens at the government rate of $91 per night. In FMCS Committees 48 addition, there are numerous other hotels in the vicinity. More information on Athens can be found at: http://www.visitathensga.com/ Parting Shot 49 Registration and more details about the workshop will be available by mid-December on the FMCS website (http://molluskconservation.org/index.html). -

Verdeca 011718 Draft Hi Yield Soy Bean EA
Verdeca Petition (17-223-01p) for Determination of Nonregulated Status for HB4 Soybean (Event IND- 00410-5) Genetically Engineered for Increased Yield and Resistance to Glufosinate-Ammonium OECD Unique Identifier: IND-00410-5 Final Environmental Assessment May 2019 Agency Contact Cindy Eck Biotechnology Regulatory Services 4700 River Road USDA, APHIS Riverdale, MD 20737 Fax: (301) 734-8669 The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’S TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326–W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250–9410 or call (202) 720–5964 (voice and TDD). USDA is an equal opportunity provider and employer. Mention of companies or commercial products in this report does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned. USDA neither guarantees nor warrants the standard of any product mentioned. Product names are mentioned solely to report factually on available data and to provide specific information. This publication reports research involving pesticides. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. i ii TABLE OF CONTENTS Page LIST OF FIGURES................................................................................................... -

Louisiana's Animal Species of Greatest Conservation Need (SGCN)
Louisiana's Animal Species of Greatest Conservation Need (SGCN) ‐ Rare, Threatened, and Endangered Animals ‐ 2020 MOLLUSKS Common Name Scientific Name G‐Rank S‐Rank Federal Status State Status Mucket Actinonaias ligamentina G5 S1 Rayed Creekshell Anodontoides radiatus G3 S2 Western Fanshell Cyprogenia aberti G2G3Q SH Butterfly Ellipsaria lineolata G4G5 S1 Elephant‐ear Elliptio crassidens G5 S3 Spike Elliptio dilatata G5 S2S3 Texas Pigtoe Fusconaia askewi G2G3 S3 Ebonyshell Fusconaia ebena G4G5 S3 Round Pearlshell Glebula rotundata G4G5 S4 Pink Mucket Lampsilis abrupta G2 S1 Endangered Endangered Plain Pocketbook Lampsilis cardium G5 S1 Southern Pocketbook Lampsilis ornata G5 S3 Sandbank Pocketbook Lampsilis satura G2 S2 Fatmucket Lampsilis siliquoidea G5 S2 White Heelsplitter Lasmigona complanata G5 S1 Black Sandshell Ligumia recta G4G5 S1 Louisiana Pearlshell Margaritifera hembeli G1 S1 Threatened Threatened Southern Hickorynut Obovaria jacksoniana G2 S1S2 Hickorynut Obovaria olivaria G4 S1 Alabama Hickorynut Obovaria unicolor G3 S1 Mississippi Pigtoe Pleurobema beadleianum G3 S2 Louisiana Pigtoe Pleurobema riddellii G1G2 S1S2 Pyramid Pigtoe Pleurobema rubrum G2G3 S2 Texas Heelsplitter Potamilus amphichaenus G1G2 SH Fat Pocketbook Potamilus capax G2 S1 Endangered Endangered Inflated Heelsplitter Potamilus inflatus G1G2Q S1 Threatened Threatened Ouachita Kidneyshell Ptychobranchus occidentalis G3G4 S1 Rabbitsfoot Quadrula cylindrica G3G4 S1 Threatened Threatened Monkeyface Quadrula metanevra G4 S1 Southern Creekmussel Strophitus subvexus -

Department of the Interior
Vol. 76 Thursday, No. 194 October 6, 2011 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; 12-Month Finding on a Petition To List Texas Fatmucket, Golden Orb, Smooth Pimpleback, Texas Pimpleback, and Texas Fawnsfoot as Threatened or Endangered; Proposed Rule VerDate Mar<15>2010 16:27 Oct 05, 2011 Jkt 226001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\06OCP2.SGM 06OCP2 mstockstill on DSK4VPTVN1PROD with PROPOSALS2 62166 Federal Register / Vol. 76, No. 194 / Thursday, October 6, 2011 / Proposed Rules DEPARTMENT OF THE INTERIOR FOR FURTHER INFORMATION CONTACT: Gary additional mussels from eastern Texas, Mowad, Texas State Administrator, U.S. the Texas heelsplitter (Potamilus Fish and Wildlife Service Fish and Wildlife Service (see amphichaenus) and Salina mucket (P. ADDRESSES); by telephone at 512–927– metnecktayi), were also included in this 50 CFR Part 17 3557; or by facsimile at 512–927–3592. petition. The petition incorporated all If you use a telecommunications device analyses, references, and documentation [FWS–R2–ES–2011–0079; MO 92210–0–0008 for the deaf (TDD), please call the provided by NatureServe in its online B2] Federal Information Relay Service database at http://www.natureserve.org/ Endangered and Threatened Wildlife (FIRS) at 800–877–8339. into the petition. Included in and Plants; 12-Month Finding on a SUPPLEMENTARY INFORMATION: NatureServe was supporting information regarding the species’ Petition To List Texas Fatmucket, Background Golden Orb, Smooth Pimpleback, taxonomy and ecology, historical and Texas Pimpleback, and Texas Section 4(b)(3)(B) of the Act (16 current distribution, present status, and Fawnsfoot as Threatened or U.S.C.