Asymbiotic Germination Bletia Purpurea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
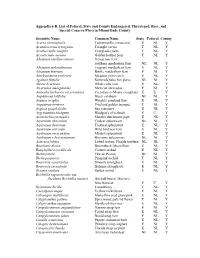
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca. -

CITES Orchid Checklist Volumes 1, 2 & 3 Combined
CITES Orchid Checklist Online Version Volumes 1, 2 & 3 Combined (three volumes merged together as pdf files) Available at http://www.rbgkew.org.uk/data/cites.html Important: Please read the Introduction before reading this Part Introduction - OrchidIntro.pdf Part I : All names in current use - OrchidPartI.pdf (this file) Part II: Accepted names in current use - OrchidPartII.pdf Part III: Country Checklist - OrchidPartIII.pdf For the genera: Aerangis, Angraecum, Ascocentrum, Bletilla, Brassavola, Calanthe, Catasetum, Cattleya, Constantia, Cymbidium, Cypripedium, Dendrobium (selected sections only), Disa, Dracula, Encyclia, Laelia, Miltonia, Miltonioides, Miltoniopsis, Paphiopedilum, Paraphalaenopsis, Phalaenopsis, Phragmipedium, Pleione, Renanthera, Renantherella, Rhynchostylis, Rossioglossum, Sophronitella, Sophronitis Vanda and Vandopsis Compiled by: Jacqueline A Roberts, Lee R Allman, Sharon Anuku, Clive R Beale, Johanna C Benseler, Joanne Burdon, Richard W Butter, Kevin R Crook, Paul Mathew, H Noel McGough, Andrew Newman & Daniela C Zappi Assisted by a selected international panel of orchid experts Royal Botanic Gardens, Kew Copyright 2002 The Trustees of The Royal Botanic Gardens Kew CITES Secretariat Printed volumes: Volume 1 first published in 1995 - Volume 1: ISBN 0 947643 87 7 Volume 2 first published in 1997 - Volume 2: ISBN 1 900347 34 2 Volume 3 first published in 2001 - Volume 3: ISBN 1 84246 033 1 General editor of series: Jacqueline A Roberts 2 Part I: ORCHIDACEAE BINOMIALS IN CURRENT USAGE Ordered alphabetically on All -

PC22 Doc. 22.1 Annex (In English Only / Únicamente En Inglés / Seulement En Anglais)
Original language: English PC22 Doc. 22.1 Annex (in English only / únicamente en inglés / seulement en anglais) Quick scan of Orchidaceae species in European commerce as components of cosmetic, food and medicinal products Prepared by Josef A. Brinckmann Sebastopol, California, 95472 USA Commissioned by Federal Food Safety and Veterinary Office FSVO CITES Management Authorithy of Switzerland and Lichtenstein 2014 PC22 Doc 22.1 – p. 1 Contents Abbreviations and Acronyms ........................................................................................................................ 7 Executive Summary ...................................................................................................................................... 8 Information about the Databases Used ...................................................................................................... 11 1. Anoectochilus formosanus .................................................................................................................. 13 1.1. Countries of origin ................................................................................................................. 13 1.2. Commercially traded forms ................................................................................................... 13 1.2.1. Anoectochilus Formosanus Cell Culture Extract (CosIng) ............................................ 13 1.2.2. Anoectochilus Formosanus Extract (CosIng) ................................................................ 13 1.3. Selected finished -

Phylogeny, Character Evolution and the Systematics of Psilochilus (Triphoreae)
THE PRIMITIVE EPIDENDROIDEAE (ORCHIDACEAE): PHYLOGENY, CHARACTER EVOLUTION AND THE SYSTEMATICS OF PSILOCHILUS (TRIPHOREAE) A Dissertation Presented in Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Erik Paul Rothacker, M.Sc. ***** The Ohio State University 2007 Doctoral Dissertation Committee: Approved by Dr. John V. Freudenstein, Adviser Dr. John Wenzel ________________________________ Dr. Andrea Wolfe Adviser Evolution, Ecology and Organismal Biology Graduate Program COPYRIGHT ERIK PAUL ROTHACKER 2007 ABSTRACT Considering the significance of the basal Epidendroideae in understanding patterns of morphological evolution within the subfamily, it is surprising that no fully resolved hypothesis of historical relationships has been presented for these orchids. This is the first study to improve both taxon and character sampling. The phylogenetic study of the basal Epidendroideae consisted of two components, molecular and morphological. A molecular phylogeny using three loci representing each of the plant genomes including gap characters is presented for the basal Epidendroideae. Here we find Neottieae sister to Palmorchis at the base of the Epidendroideae, followed by Triphoreae. Tropidieae and Sobralieae form a clade, however the relationship between these, Nervilieae and the advanced Epidendroids has not been resolved. A morphological matrix of 40 taxa and 30 characters was constructed and a phylogenetic analysis was performed. The results support many of the traditional views of tribal composition, but do not fully resolve relationships among many of the tribes. A robust hypothesis of relationships is presented based on the results of a total evidence analysis using three molecular loci, gap characters and morphology. Palmorchis is placed at the base of the tree, sister to Neottieae, followed successively by Triphoreae sister to Epipogium, then Sobralieae. -

E Edulis ATION FLORISTIC ELEMENTS AS BASIS FOR
Oecologia Australis 23(4):744-763, 2019 https://doi.org/10.4257/oeco.2019.2304.04 GEOGRAPHIC DISTRIBUTION OF THE THREATENED PALM Euterpe edulis Mart. IN THE ATLANTIC FOREST: IMPLICATIONS FOR CONSERVATION FLORISTIC ELEMENTS AS BASIS FOR CONSERVATION OF WETLANDS AND PUBLIC POLICIES IN BRAZIL: THE CASE OF VEREDAS OF THE PRATA RIVER Aline Cavalcante de Souza1* & Jayme Augusto Prevedello1 1 1 1,2 1 1 Universidade do Estado do Rio de Janeiro, Instituto de Biologia, Departamento de Ecologia, Laboratório de Ecologia de Arnildo Pott *,Vali Joana Pott , Gisele Catian & Edna Scremin-Dias Paisagens, Rua São Francisco Xavier 524, Maracanã, CEP 20550-900, Rio de Janeiro, RJ, Brazil. 1 Universidade Federal de Mato Grosso do Sul, Instituto de Biociências, Programa de Pós-Graduação Biologia Vegetal, E-mails: [email protected] (*corresponding author); [email protected] Cidade Universitária, s/n, Caixa Postal 549, CEP 79070-900, Campo Grande, MS, Brazil. 2 Universidade Federal de Mato Grosso, Departamento de Ciências Biológicas, Cidade Universitária, Av. dos Estudantes, Abstract: The combination of species distribution models based on climatic variables, with spatially explicit 5055, CEP 78735-901, Rondonópolis, MT, Brazil. analyses of habitat loss, may produce valuable assessments of current species distribution in highly disturbed E-mails: [email protected] (*corresponding author); [email protected]; [email protected]; ecosystems. Here, we estimated the potential geographic distribution of the threatened palm Euterpe [email protected] edulis Mart. (Arecaceae), an ecologically and economically important species inhabiting the Atlantic Forest biodiversity hotspot. This palm is shade-tolerant, and its populations are restricted to the interior of forest Abstract: Vereda is the wetland type of the Cerrado, often associated with the buriti palm Mauritia flexuosa. -

Yaxha, Peten, Guatemala
AQUATIC ORCHIDS? Bletia purpurea Yaxha, Peten, Guatemala DR. NICHOLAS HELLMUTH AQUATIC ORCHIDS? December 2018 MAIN AUTHOR Nicholas Hellmuth FLAAR (USA) FLAAR Mesoamérica (Guatemala) EDITION Vivian Díaz Marcella Sarti Flor Setina COVER PHOTOGRAPH Nicholas Hellmuth INTERNAL PHOTOGRAPHS Nicholas Hellmuth ART DIRECTION Andrea Sánchez LAYOUT Andrea Sánchez This report was made in cooperation with the COVER PHOTOGRAPH: Yaxha-Nakum-Naranjo National Park to help Bletia purpurea promove the park, its natural resources and, Nikon D810. Lente Nikon 200mm f/4 AF-D Macro, f/16, 1/640, ISO 1250. Yaxha, Petén, Guatemala. Photography by: Nicholas attract future visitors. Hellmuth, FLAAR Mesoamérica. INDEX PHOTOGRAPH: Bletia purpurea Nikon D810. Lente Nikon 200mm f/4 AF-D Macro, f/16, 1/640, ISO 1000. Yaxha, Petén, Guatemala.Photography by: Nicholas Hellmuth, FLAAR Mesoamérica. 3 FLAAR Mesoamérica (Foundation for Latin American Anthropo- logical Research), is a nonprofit Guatemalan institution founded under the direction and enthusiasm of Dr. Nicholas Hellmuth, Classic Mayan Art specialist, with the aim of wanting to see our country to be recognized throughout the world for its land- scapes, culture and natural resources. We believe knowledge and ancestral wisdom of natural resources can be taken to any kind of person through education. At the same time, it will awake admiration and desire in people who follow our work to preserve these resources. One of our main objectives is to create consciousness about looking after Mesoamerica natural diversity. Thus, the FLAAR team creates educational material to raise public awareness of it. The work done in FLAAR Mesoamerica consists on the methodological compilation of facts about nature, flora, fauna, history, and culture of Mesoamerica, and to spread it up to the general public who play an important role in the conservation of ecosystems. -

Table S2. Occurrence Data and List of Species Used for Species Distribution Modeling (SDM)
Table S2. Occurrence data and list of species used for species distribution modeling (SDM). spatially unique occurrence Species points Allium eurotophilum 5 Allium glandulosum 87 Allium haematochiton 8 Alophia drummondii 7 Arisaema macrospathum 12 Beloglottis costaricensis 7 Bessera elegans 15 Bletia adenocarpa 21 Bletia campanulata 55 Bletia coccinea 21 Bletia ensifolia 17 Bletia gracilis 29 Bletia lilacina 8 Bletia macristhmochila 29 Bletia neglecta 26 Bletia parkinsonii 22 Bletia punctata 20 Bletia purpurata 34 Bletia purpurea 108 Bletia roezlii 38 Bletia tenuifolia 6 Brachystele polyantha 8 Calochortus barbatus 18 Calochortus exilis 5 Calochortus fuscus 7 Calochortus purpureus 7 Calochortus spatulatus 8 Calochortus venustulus 6 Cardiostigma 8 longispatha Cipura campanulata 7 Cipura paludosa 15 Corallorhiza bulbosa 5 Crinum americanum 7 Cypripedium irapeanum 11 Cypripedium molle 9 Deiregyne densiflora 8 Dichromanthus 15 cinnabarinus Dichromanthus 14 michuacanus Echeandia 9 echeandioides Echeandia flavescens 11 Echeandia flexuosa 9 Echeandia 5 longipedicellata Echeandia luteola 6 Echeandia mexicana 16 Echeandia nana 7 Echeandia occidentalis 10 Echeandia paniculata 9 Echeandia parviflora 12 Echeandia ramosissima 9 Echeandia reflexa 5 Echeandia scabrella 6 Echeandia skinneri 18 Echeandia vestita 17 Eleutherine bulbosa 5 Eleutherine latifolia 7 Govenia alba 6 Govenia liliacea 14 Govenia mutica 6 Govenia purpusii 7 Govenia superba 12 Habenaria clypeata 20 Habenaria novemfida 9 Habenaria strictissima 5 Habranthus longifolius 5 Hexalectris -

Native Orchid Conservation, Florida Panther National Wildlife Refuge
Native Orchid Conservation on the Florida Panther National Wildlife Refuge: A Review of Cooperative Research, Posed Questions, and Recommendations 2011 Florida Orchid Conservation Conference Naples Botanical Garden, Naples, Florida December 2-3, 2011 TABLE OF CONTENTS INTRODUCTION ............................................................................................ 2 COMMONLY USED APPROACHES FOR ORCHID CONSERVATION RESEARCH .......... 3 PRESENTATION ABSTRACTS .......................................................................... 5 SPECIES SPECIFIC RESEARCH BLETIA PURPUREA, PINE-PINK ............................................................................ 7 CALOPOGON TUBEROSUS, COMMON GRASS-PINK ...................................................... 9 CYRTOPODIUM PUNCTATUM, CIGAR ORCHID .......................................................... 12 DENDROPHYLAX LINDENII, GHOST ORCHID ........................................................... 14 EPIDENDRUM NOCTURNUM, NIGHT-FRAGRANT EPIDENDRUM ....................................... 15 EULOPHIA ALTA, WILD COCO ........................................................................... 16 NON-SPECIES SPECIFIC RESEARCH .............................................................. 18 SCIENTIFIC MANUSCRIPT BIBLIOGRAPHY ...................................................... 19 CONSERVATION STRATEGIES QUESTIONS TO CONSIDER................................ 22 Prepared by James J. Sadler, a current graduate assistant in the Environmental Horticulture Department, University of Florida -

Ethnobotany, Pharmacology and Chemistry of Medicinal Orchids from Veracruz
Journal of Agricultural Science and Technology A 5 (2015) 745-754 doi: 10.17265/2161-6256/2015.09.006 D DAVID PUBLISHING Ethnobotany, Pharmacology and Chemistry of Medicinal Orchids from Veracruz Leticia Margarita Cano Asseleih, Rebeca Alicia Menchaca García and José Yader Sageth Ruiz Cruz Centro de Investigaciones Tropicales (CITRO), Universidad Veracruzana, Xalapa 91000, Mexico Abstract: Orchidaceae is a large family of 1,260 species in Mexico, of which 433 grow in the state of Veracruz, Mexico. Although economically important in horticulture because of the beauty of their flowers, researches have done little work regarding their medicinal properties. This paper aimed to present the results of ethnobotanical, pharmacological and active compounds research on Veracruz medicinal orchids. The ethnobotanical information was obtained by consulting the Atlas of the Mexican Traditional Medicine Plants, Veracruz Medicinal Flora Database (CITRO-UV project) and through field work in the Nahuatl community of Cuautlajapa, Veracruz. To obtain pharmacological and active compounds information of registered species, a search was carried out through MEDLINE (USA National Library of Medicine Journal Citation database). Twelve medicinal orchids were recorded for Veracruz, i.e., Epidendrum chlorocorymbos Schltr., Habenaria floribunda Lindl., Isochillus latibracteatus A. Rich. & Galeotti, Isochillus major Schltdl. & Cham., Mormodes maculata var. unicolor (Hook.) L. O. Williams, Oestlundia luteorosea (A. Rich. & Galeotti) W. E. Higgins, Oncidium ascendens Lindl., Scaphyglottis fasciculata Hook., Sobralia macrantha Lindl., Spiranthes eriophora (Rob. & Greenm.), Stanhopea oculata (G. Lodd.) Lindl. and Vanilla planifolia Andrews. Only two species have been investigated in terms of their pharmacology and active compounds. Also, information for another five species closely related to already identified ones was obtained. -

Johnson 2011 Light Temperature Bletia Purpurea
Plant Growth Regul (2011) 63:89–99 DOI 10.1007/s10725-010-9516-3 ORIGINAL RESEARCH Examining the interaction of light, nutrients and carbohydrates on seed germination and early seedling development of Bletia purpurea (Orchidaceae) Timothy R. Johnson • Michael E. Kane • Hector E. Pe´rez Received: 14 April 2010 / Accepted: 19 August 2010 / Published online: 5 September 2010 Ó Springer Science+Business Media B.V. 2010 Abstract The effects of carbohydrate availability, Keywords Nutrition Á Photoblastic Á Rhizoid Á carbohydrate source, nutrient availability and illumination Seed physiology Á Seedling development on germination and early development of Bletia purpurea (Orchidaceae) seeds were investigated using asymbiotic seed germination. Of special interest was determining the Introduction minimum nutritional and light requirements for the com- pletion of germination. Germination and development was In nature, germination of orchid seeds is dependent upon limited when seeds were cultured in darkness without the formation of a symbiotic relationship with fungi, which sucrose. Seeds were able to germinate under illuminated supply nutrients and carbohydrates to the minute, non- conditions even in the absence of sucrose and this effect endospermic seeds and rudimentary embryo (Alexander was enhanced when mineral nutrients were incorporated et al. 1984; Alexander and Hadley 1985; Manning and van into media. Sucrose, fructose, glucose and trehalose Staden 1987; McKendrick et al. 2000; Rasmussen 1995; enhanced germination and seedling development while Yoder et al. 2000). This relationship can be replicated in mannitol and sorbitol did not. These data suggest that vitro by co-culturing orchid seeds with compatible fungi carbohydrates, either as products of photosynthesis, from (symbiotic seed germination) and has been demonstrated symbiotic fungi in situ or as exogenously supplied sugars with many different orchid species (Batty et al. -

Bibliografía Botánica Del Caribe I
Consolidated bibliography Introduction To facilitate the search through the bibliographies prepared by T. Zanoni (Bibliographía botánica del Caribe, Bibliografía de la flora y de la vegetatíon de la isla Española, and the Bibliography of Carribean Botany series currently published in the Flora of Greater Antilles Newsletter), the html versions of these files have been put together in a single pdf file. The reader should note the coverage of each bibliography: Hispaniola is exhaustively covered by all three bibliographies (from the origin up to now) while other areas of the Carribean are specifically treated only since 1984. Please note that this pdf document is made from multiple documents, this means that search function is called by SHIFT+CTRL+F (rather than by CTRL+F). Please let me know of any problem. M. Dubé The Jardín Botánico Nacional "Dr. Rafael M. Moscoso," Santo Domingo, Dominican Republic, publishers of the journal Moscosoa, kindly gave permission for the inclusion of these bibliographies on this web site. Please note the present address of T. Zanoni : New York Botanical Garden 200th Street at Southern Blvd. Bronx, NY 10458-5126, USA email: [email protected] Moscosoa 4, 1986, pp. 49-53 BIBLIOGRAFÍA BOTÁNICA DEL CARIBE. 1. Thomas A. Zanoni Zanoni. Thomas A. (Jardín Botánico Nacional, Apartado 21-9, Santo Domingo, República Dominicana). Bibliografía botánica del Caribe. 1. Moscosoa 4: 49-53. 1986. Una bibliografía anotada sobre la literatura botánica publicada en los años de 1984 y 1985. Se incluyen los temas de botánica general y la ecología de las plantas de las islas del Caribe. An annotated bibliography of the botanical literature published in 1984 and 1985, covering all aspects of botany and plant ecology of the plants of the Caribbean Islands.