Virginia: in the Circuit Court of Henry County
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DCAT MEMBER COMPANY MEETING LOCATOR V. 4
DCAT MEMBER COMPANY MEETING LOCATOR v. 4 THE BENJAMIN Sancilio Pharmaceuticals Company, Inc. Sri Krishna Pharmaceuticals Ltd. Amino Chemicals Ltd. Zydus Pharmaceuticals (USA) Inc. Apogee Pharma, Inc. C2 PHARMA INTERCONTINENTAL BARCLAY Calyx Chemicals & Pharmaceuticals Ltd. AbbVie* ChemCon GmbH ACIC Pharmaceuticals Inc. Concord Biotech Limited Advitech SA Dipharma Francis Srl Amneal Pharmaceuticals LLC DSM Sinochem Pharmaceuticals ALP Pharm F.I.S. - Fabbrica Italiana Sintetici S.p.A. AMRI Jost Chemical Co. Asymchem Inc. Jubilant Pharma Capsugel, Now a Lonza Company* PharmSource, A GlobalData Company CBC AMERICAS Corp. PolyPeptide Group CellMark USA, LLC ROHNER Inc. Charioteer Pharmaceutical Co., Ltd., Zhejiang FIFTY NYC, A AFFINA HOTEL Chemical and Pharmaceutical Solutions Chiral Quest Corp. Chartwell Pharmaceuticals, LLC Croda, Inc. HOTEL 48LEX DFE Pharma AB BioTechnologies, Inc. DPL-US AiPing Pharmaceutical, Inc. EQ Esteve Almac Evonik Corporation Aptuit LLC FAREVA SA AZAD Fine Chemicals Ltd. Flavine North America, Inc. Cambridge Isotope Laboratories, Inc. Formosa Laboratories, Inc. CMC Biologics Grifols International S.A. Groupe Parima Hainan Poly Pharm. Co., Ltd. Navin Fluorine International Limited Harris Pharmaceutical Qualicaps, Inc. Helm AG RC2 Pharma Connect LLC Hetero USA, Inc. Recipharm Hikal, Ltd. Recro Gainesville LLC Interchem Corporation Reed-Lane, Inc. Inventia Healthcare PVT LTD Please note: Some DCAT member companies have requested not to be listed in the locator. (*) indicates member companies with Business Meeting Spaces in more than one hotel. INTERCONTINENTAL BARCLAY CONT'D PiSA BioPharm, Inc. SPI Pharma Inc. Johnson Matthey Tapemark Kingchem Life Science LLC Unither Pharmaceutical Legacy Pharmaceutical Packaging Uquifa S.A. Lonza AG* Neuland Laboratories Ltd. LOTTE NY PALACE Orion Group AbbVie* Par Pharmaceutical, Inc. -

BTG INTERNATIONAL LIMITED V. AMNEAL PHARMACEUTICALS LLC
United States Court of Appeals for the Federal Circuit ______________________ BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMNEAL PHARMACEUTICALS LLC, AMNEAL PHARMACEUTICALS OF NEW YORK, LLC, DR. REDDY'S LABORATORIES, INC., DR. REDDY'S LABORATORIES, LTD., WOCKHARDT BIO AG, WOCKHARDT USA LLC, WOCKHARDT LTD., MYLAN PHARMACEUTICALS INC., MYLAN INC., WEST-WARD PHARMACEUTICALS CORP., NKA HIKMA PHARMACEUTICALS USA INC., HIKMA PHARMACEUTICALS LLC, TEVA PHARMACEUTICALS USA, INC., Defendants-Appellees PAR PHARMACEUTICAL, INC., PAR PHARMACEUTICAL COMPANIES, INC., RISING PHARMACEUTICALS, INC., Defendants ______________________ 2019-1147 ______________________ Appeal from the United States District Court for the District of New Jersey in Nos. 2:15-cv-05909-KM-JBC, 2:16-cv-02449-KM-JBC, 2:17-cv-06435-KM-JBC, Judge Kevin McNulty. 2 BTG INTERNATIONAL LIMITED v. AMNEAL PHARMACEUTICALS LLC - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMERIGEN PHARMACEUTICALS, INC., AMERIGEN PHARMACEUTICALS LIMITED, Defendants-Appellees ______________________ 2019-1148 ______________________ Appeal from the United States District Court for the District of New Jersey in No. 2:16-cv-02449-KM-JBC, Judge Kevin McNulty. - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - JANSSEN -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Complaint 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
1 Geoffrey D. Strommer (AK Bar # 0911044) Dawn E. Winalski (AK Bar # 1311107) 2 Edmund Clay Goodman (pro hac vice admission pending) Hobbs, Straus, Dean & Walker, LLP 3 516 SE Morrison Street, Suite 1200 4 Portland, OR 97214 Phone: (503) 2421745 5 Fax: (503) 2421072 Email: [email protected] 6 Email: [email protected] 7 Email: [email protected] 8 Attorneys for SouthEast Alaska Regional Health Consortium 9 10 11 UNITED STATES DISTRICT COURT 12 DISTRICT OF ALASKA 13 14 SOUTHEAST ALASKA REGIONAL Case No.: 3:18-cv-00217-TMB HEALTH CONSORTIUM, 15 COMPLAINT 16 Plaintiff, JURY TRIAL DEMANDED 17 vs. 18 PURDUE PHARMA L.P.; PURDUE 19 PHARMA INC.; THE PURDUE FREDERICK COMPANY; RHODES 20 PHARMACEUTICALS, L.P.; RHODES TECHNOLOGIES, INC.; CEPHALON, 21 INC.; TEVA PHARMACEUTICAL 22 INDUSTRIES, LTD.; TEVA PHARMACEUTICALS USA, INC; 23 JANSSEN PHARMACEUTICALS, INC.; ORTHOMCNEILJANSSEN 24 PHARMACEUTICALS, INC. N/K/A 25 JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC. 26 N/K/A JANSSEN PHARMACEUTICALS, INC.; NORAMCO, INC.; ENDO HEALTH 27 SOLUTIONS INC.; ENDO COMPLAINT Case 3:18-cv-00217-TMB Document 1 Filed 09/20/18 Page 1 of 163 1 PHARMACEUTICALS INC.; ENDO INTERNATIONAL PLC; PAR 2 PHARMACEUTICAL, INC.; PAR PHARMACEUTICALS COMPANIES, 3 INC. F/K/A PAR PHARMACEUTICAL 4 HOLDINGS, INC.; ALLERGAN PLC F/K/A ACTAVIS PLC; ALLERGAN 5 FINANCE LLC, F/K/A ACTAVIS, INC., F/K/A WATSON PHARMACEUTICALS, 6 INC.; WATSON LABORATORIES, INC.; 7 ACTAVIS LLC; ACTAVIS PHARMA, INC. F/K/A WATSON PHARMA, INC.; 8 INSYS THERAPEUTICS, INC.; MALLINCKRODT PLC; 9 MALLINCKRODT, LLC; SPECGX LLC; 10 ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC; AMNEAL 11 PHARMACEUTICALS, INC F/K/A AMNEAL PHARMACEUTICALS, LLC; 12 KVKTECH, INC.; MCKESSON CORP.; 13 CARDINAL HEALTH, INC.; CARDINAL HEALTH 110, LLC; 14 AMERISOURCEBERGEN CORP.; ANDA, INC.; ANDA PHARMACEUTICALS, 15 INC.; HENRY SCHEIN, INC.; HENRY 16 SCHEIN MEDICAL SYSTEMS, INC.; and JOHN & JANE DOES 1100 INCLUSIVE, 17 Defendants. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Opioid Prescriptions Filled in The
VIRGINIA: IN THE CIRCUIT COURT FOR WISE COUNTY AND THE CITY OF NORTON CITY OF NORTON, VIRGINIA, Plaintiff, v. PURDUE PHARMA, L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; RHODES PHARMACEUTICALS, L.P.; ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC.; MALLINCKRODT PLC; MALLINCKRODT LLC; ENDO HEALTH SOLUTIONS, INC; ENDO Case No. CL18 - __________ PHARMACEUTICALS, INC.; PAR PHARMACEUTICAL COMPANIES, INC.; Jury Trial Demanded PAR PHARMACEUTICAL, INC.; TEVA PHARMACEUTICALS USA, INC.; CEPHALON, INC.; BARR LABORATORIES, INC.; JANSSEN PHARMACEUTICALS, INC.; ORTHO- MCNEIL-JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC.; WATSON LABORATORIES, INC.; ALLERGAN PLC; ACTAVIS PHARMA, INC.; ACTAVIS, LLC; INSYS THERAPEUTICS, INC.; KVK-TECH, INC.; AMNEAL PHARMACEUTICALS LLC; IMPAX LABORATORIES, LLC; AMNEAL PHARMACEUTICALS, INC.; AMNEAL PHARMACEUTICALS OF NEW YORK, LLC; MYLAN PHARMACEUTICALS, INC.; MCKESSON CORPORATION; MCKESSON MEDICAL-SURGICAL INC.; CARDINAL HEALTH, INC.; AMERISOURCEBERGEN DRUG CORPORATION; HENRY SCHEIN, INC.; GENERAL INJECTABLES & VACCINES, INC.; INSOURCE, INC.; CVS HEALTH CORPORATION; CVS PHARMACY, INC.; CVS TN DISTRIBUTION, L.L.C.; WALGREENS BOOTS ALLIANCE, INC.; WALGREEN CO.; EXPRESS SCRIPTS HOLDING COMPANY; EXPRESS SCRIPTS, INC; CAREMARK RX, L.L.C.; CAREMARKPCS HEALTH, L.L.C.; CAREMARK, L.L.C.; UNITEDHEALTH GROUP INCORPORATED; OPTUM, INC.; OPTUMRX, INC.; and DOES 1-100, Defendants. ii PLAINTIFF’S ORIGINAL COMPLAINT Plaintiff, the City of Norton, Virginia, by and through the undersigned attorneys, (hereinafter -

Teva Pharmaceuticals
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF CONNECTICUT THE STATE OF CONNECTICUT; THE STATE OF ALABAMA; THE STATE OF ALASKA; Civil Action No. THE STATE OF ARIZONA; THE STATE OF COLORADO; THE STATE OF DELAWARE; THE STATE OF FLORIDA; THE STATE OF HAWAII; THE STATE OF IDAHO; May 10, 2019 THE STATE OF ILLINOIS; THE STATE OF INDIANA; THE STATE OF IOWA; COMPLAINT THE STATE OF KANSAS; THE COMMONWEALTH OF KENTUCKY; THE STATE OF LOUISIANA; THE STATE OF MAINE; THE STATE OF MARYLAND; Non-Public Version: Filed Under Seal THE COMMONWEALTH OF MASSACHUSETTS; THE STATE OF MICHIGAN; THE STATE OF MINNESOTA; THE STATE OF MISSISSIPPI; THE STATE OF MISSOURI; THE STATE OF MONTANA; THE STATE OF NEBRASKA; THE STATE OF NEVADA; THE STATE OF NEW JERSEY; THE STATE OF NEW MEXICO; THE STATE OF NEW YORK; THE STATE OF NORTH CAROLINA; THE STATE OF NORTH DAKOTA; THE STATE OF OHIO; THE STATE OF OKLAHOMA; THE STATE OF OREGON; THE COMMONWEALTH OF PENNSYLVANIA; THE COMMONWEALTH OF PUERTO RICO; THE STATE OF RHODE ISLAND; THE STATE OF SOUTH CAROLINA; THE STATE OF TENNESSEE; THE STATE OF UTAH; THE STATE OF VERMONT; THE COMMONWEALTH OF VIRGINIA; THE STATE OF WASHINGTON; THE STATE OF WEST VIRGINIA; THE STATE OF WISCONSIN; v. TEVA PHARMACEUTICALS USA, INC.; ACTAVIS HOLDCO US, INC.; ACTAVIS PHARMA, INC.; AMNEAL PHARMACEUTICALS, INC.; APOTEX CORP.; ARA APRAHAMIAN; AUROBINDO PHARMA U.S.A., INC.; DAVID BERTHOLD; BRECKENRIDGE PHARMACEUTICAL, INC.; JAMES (JIM) BROWN; MAUREEN CAVANAUGH; TRACY SULLIVAN DIVALERIO; DR. REDDY'S LABORATORIES, INC.; MARC FALKIN; GLENMARK PHARMACEUTICALS, INC., USA; JAMES (JIM) GRAUSO; KEVIN GREEN; GREENSTONE LLC; ARMANDO KELLUM; LANNETT COMPANY, INC.; LUPIN PHARMACEUTICALS, INC.; MYLAN PHARMACEUTICALS INC.; JILL NAILOR; JAMES (JIM) NESTA; PAR PHARMACEUTICAL COMPANIES, INC.; NISHA PATEL; PFIZER, INC.; KONSTANTIN OSTAFICIUK; DAVID REKENTHALER; RICHARD (RICK) ROGERSON; SANDOZ, INC.; TARO PHARMACEUTICALS USA, INC. -
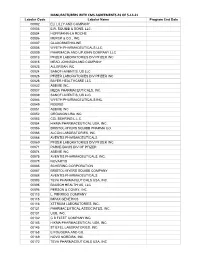
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC. -

Download Current Complete HIN Authorized Licensee List
Health Industry Business Communications Council Health Industry Number System (HIN ®) Authorized Licensees Fresenius Kabi USA, Inc. The following companies (and/ Manufacturers Abbott Laboratories Fresenius Medical Care or their subsidiaries/divisions) AbbVie G1 Therapeutics, Inc. and organizations are authorized Acadia Pharmaceuticals GE Healthcare Genentech, Inc. licensees that have access to the Acrotech Biopharma LLC Akorn Pharmaceuticals Roche Labs Health Industry Number (HIN) Alexion Pharmaceuticals, Inc. Gilead Sciences, Inc. System Database, or a portion Alkermes Inc. Glaxo SmithKline, Inc. Glenmark Pharmaceuticals Inc. thereof. Allergan, Inc. Alvogen Inc Grifols USA, LLC Ambu Inc GyrusACMI dba Olympus-OSTA HIN licenses do not convey Amgen, Inc. Hikma Horizon Therapeutics USA, Inc. between a parent organization Amneal Pharmaceuticals, LLC Amphastar Pharmaceuticals, Inc. HPSRx Enterprises, Inc. and/or its subsidiaries and Apotex Corporation ICU Medical divisions. Each subsidiary and Argon Medical Devices, Inc. Incyte Ipsen Biopharmaceuticals Inc. division must maintain its own, Arjo Inc. Ascensia Diabetes Care Jazz Pharmaceuticals individual license to the HIN Astra Zeneca Johnson & Johnson Health Care Sys. Database in order to be an Aurobindo Pharma USA Advanced Sterilization Products Centocor Authorized Licensee AuroMedics Pharma LLC B. Braun Medical Inc. Cordis Corportion Bausch Health US, LLC. DuPuy Orthopaedics, Inc. Licensees have access to Bausch & Lomb Surgical Ethicon, Inc. Ethicon Endo Surgery, Inc. more than 1,000,000 customer Bavarian Nordic, Inc. Baxter Healthcare Corporation J&J Consumer Products location records and related Bayer Healthcare Pharmaceuticals J&J Merck Consumer Pharm. HIN assignments for use in Beckman Coulter, Inc. Janssen Pharma. Products, LP Lifescan, Inc. electronic commerce, customer Becton Dickinson Biogen Idec McNeil Consumer Healthcare Co. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Amneal and Impax, in the United States: Heritage Pharmaceuticals, Inc., Sandoz (A Subsidiary of Novartis AG), and Teva Pharmaceutical Industries Ltd
ANALYSIS OF AGREEMENT CONTAINING CONSENT ORDERS TO AID PUBLIC COMMENT In the Matter of Amneal Holdings, LLC, Amneal Pharmaceuticals LLC, Impax Laboratories, Inc., and Impax Laboratories, LLC File No. 181-0017 The Federal Trade Commission (“Commission”) has accepted, subject to final approval, an Agreement Containing Consent Orders (“Consent Agreement”) from Amneal Holdings, LLC, Amneal Pharmaceuticals LLC (collectively, “Amneal”), Impax Laboratories, Inc., and Impax Laboratories, LLC (collectively, “Impax”) that is designed to remedy the anticompetitive effects resulting from Amneal’s acquisition of equity interests of Impax. Under the terms of the proposed Consent Agreement, the parties are required to divest all of Impax’s rights and assets related to the following seven products to ANI Pharmaceuticals, Inc. (“ANI”): generic desipramine hydrochloride tablets; generic felbamate tablets; generic aspirin and dipyridamole extended release (“ER”) capsules; generic diclofenac sodium and misoprostol delayed release (“DR”) tablets; generic ezetimibe and simvastatin immediate release (“IR”) tablets; generic erythromycin tablets; and generic methylphenidate hydrochloride ER tablets. Pursuant to the Consent Agreement, the parties also are required to divest all of Impax’s rights and assets related to generic azelastine nasal spray and generic olopatadine hydrochloride nasal spray to Perrigo Company plc (“Perrigo”), and to divest all of Impax’s rights and assets related to generic fluocinonide-E cream to G&W Laboratories (“G&W”). The proposed Consent Agreement has been placed on the public record for thirty days for receipt of comments from interested persons. Comments received during this period will become part of the public record. After thirty days, the Commission will again evaluate the proposed Consent Agreement, along with the comments received, to make a final decision as to whether it should withdraw from the proposed Consent Agreement, modify it, or make final the Decision and Order (“Order”).