Alkaloids Derived from Tryptophan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Serotonin Functioning and Adolescents' Alcohol
Development and Psychopathology, 2017, page 1 of 21 # Cambridge University Press 2017 doi:10.1017/S095457941700058X Serotonin functioning and adolescents’ alcohol use: A genetically informed study examining mechanisms of risk FRANCES L. WANG,a LAURIE CHASSIN,a JOHN E. BATES,b DANIELLE DICK,c JENNIFER E. LANSFORD,d e d GREGORY S. PETTIT, AND KENNETH A. DODGE aArizona State University; bIndiana University Bloomington; cVirginia Commonwealth University; dDuke University; and eAuburn University Abstract The current study used data from two longitudinal samples to test whether self-regulation, depressive symptoms, and aggression/antisociality were mediators in the relation between a polygenic score indexing serotonin (5-HT) functioning and alcohol use in adolescence. The results from an independent genome-wide association study of 5-hydroxyindoleacetic acid in the cerebrospinal fluid were used to create 5-HT polygenic risk scores. Adolescents and/or parents reported on adolescents’ self-regulation (Time 1), depressive symptoms (Time 2), aggression/antisociality (Time 2), and alcohol use (Time 3). The results showed that 5-HT polygenic risk did not predict self-regulation. However, adolescents with higher levels of 5-HT polygenic risk showed greater depression and aggression/antisociality. Adolescents’ aggression/antisociality mediated the relation between 5-HT polygenic risk and later alcohol use. Deficits in self- regulation also predicted depression and aggression/antisociality, and indirectly predicted alcohol use through aggression/antisociality. Pathways to alcohol use were especially salient for males from families with low parental education in one of the two samples. The results provide insights into the longitudinal mechanisms underlying the relation between 5-HT functioning and alcohol use (i.e., earlier aggression/antisociality). -

Tryptophan and 5-Hydroxytryptophan for Depression (Review)
Cochrane Database of Systematic Reviews Tryptophan and 5-Hydroxytryptophan for depression (Review) Shaw KA, Turner J, Del Mar C Shaw KA, Turner J, Del Mar C. Tryptophan and 5-Hydroxytryptophan for depression. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No.: CD003198. DOI: 10.1002/14651858.CD003198. www.cochranelibrary.com Tryptophan and 5-Hydroxytryptophan for depression (Review) Copyright © 2010 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 3 RESULTS....................................... 4 DISCUSSION ..................................... 4 AUTHORS’CONCLUSIONS . 5 ACKNOWLEDGEMENTS . 5 REFERENCES ..................................... 6 CHARACTERISTICSOFSTUDIES . 10 DATAANDANALYSES. 15 Analysis 1.1. Comparison 1 L-Tryptophan and 5-HTP versus placebo for the treatment of depression, Outcome 1 Numbers ofresponders................................... 15 Analysis 2.1. Comparison 2 Side-effects of L-Tryptophan and 5-HTP versus placebo, Outcome 1 Numbers with side- effects. .................................... 16 FEEDBACK...................................... 16 WHAT’SNEW..................................... 16 HISTORY....................................... 17 CONTRIBUTIONSOFAUTHORS . 17 DECLARATIONSOFINTEREST . 17 SOURCESOFSUPPORT -

Effects of the Putative Antipsychotic Alstonine on Glutamate Uptake in Acute Hippocampal Slices ⇑ Ana P
Neurochemistry International 61 (2012) 1144–1150 Contents lists available at SciVerse ScienceDirect Neurochemistry International journal homepage: www.elsevier.com/locate/nci Effects of the putative antipsychotic alstonine on glutamate uptake in acute hippocampal slices ⇑ Ana P. Herrmann a,b, , Paula Lunardi b, Luísa Klaus Pilz a, Ana C. Tramontina b, Viviane M. Linck a,b, Christopher O. Okunji c, Carlos A. Gonçalves b, Elaine Elisabetsky a,b a Laboratório de Etnofarmacologia, Departamento de Farmacologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Rua Sarmento Leite, 500, 90050-170 Porto Alegre, RS, Brazil b Programa de Pós-graduação em Ciências Biológicas: Bioquímica, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Rua Ramiro Barcelos, 2600, 90035-000 Porto Alegre, RS, Brazil c International Centre for Ethnomedicine and Drug Development (InterCEDD), Nsukka, Enugu State, Nigeria article info abstract Article history: A dysfunctional glutamatergic system is thought to be central to the negative symptoms and cognitive Received 8 March 2012 deficits recognized as determinant to the poor quality of life of people with schizophrenia. Modulating Received in revised form 13 August 2012 glutamate uptake has, thus, been suggested as a novel target for antipsychotics. Alstonine is an indole Accepted 15 August 2012 alkaloid sharing with atypical antipsychotics the profile in animal models relevant to schizophrenia, Available online 25 August 2012 though divergent in its mechanism of action. The aim of this study was to evaluate the effects of alstonine on glutamate uptake. Additionally, the effects on glutathione content and extracellular S100B levels were Keywords: assessed. -

Treating Smoking Dependence in Depressed Alcoholics
Treating Smoking Dependence in Depressed Alcoholics Nassima Ait-Daoud, M.D.; Wendy J. Lynch, Ph.D.; J. Kim Penberthy, Ph.D.; Alison B. Breland, Ph.D.; Gabrielle R. Marzani-Nissen, M.D.; and Bankole A. Johnson, D.Sc., M.D., Ph.D. Alcoholism and nicotine dependence share many neurobiological underpinnings; the presence of one drug can cause a person to crave the other. Depressive illness can complicate comorbid alcohol and nicotine dependence by exacerbating the negative affect encountered during attempts to abstain from one or both drugs. Given the morbidity and mortality associated with cigarette smoking, it is imperative to identify treatments to promote smoking cessation and address comorbid psychiatric conditions contemporaneously. Pharmacotherapeutic options demonstrating varying degrees of efficacy and promise in preclinical and clinical studies include nicotine replacement therapy (NRT), selective serotonin reuptake inhibitors (SSRIs), bupropion, varenicline, tricyclic antidepressants, and bupropion plus NRT. Topiramate has shown potential for promoting smoking cessation in alcoholics, although its safety in depressed patients has not been fully explored. The efficacy of medications for treating nicotine dependence is generally enhanced by the inclusion of behavioral interventions such as cognitive behavioral therapy. When group cohesion and social support are stressed, success rates increase among depressed smokers undergoing smoking cessation treatment. Additional treatment strategies targeting dually dependent individuals with -
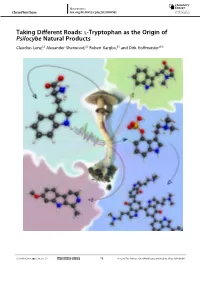
L‐Tryptophan As the Origin of Psilocybe Natural Products
Minireviews ChemPlusChem doi.org/10.1002/cplu.202000581 1 2 3 Taking Different Roads: l-Tryptophan as the Origin of 4 5 Psilocybe Natural Products 6 [a] [b] [b] [a] 7 Claudius Lenz, Alexander Sherwood, Robert Kargbo, and Dirk Hoffmeister* 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 ChemPlusChem 2021, 86, 28–35 28 © 2020 The Authors. ChemPlusChem published by Wiley-VCH GmbH Wiley VCH Mittwoch, 30.12.2020 2101 / 181786 [S. 28/35] 1 Minireviews ChemPlusChem doi.org/10.1002/cplu.202000581 1 Psychotropic fungi of the genus Psilocybe, colloquially referred highlighted. Psilocybin and its congeners, the heterogeneous 2 to as „magic mushrooms”, are best known for their l- blue-colored psilocyl oligomers, alongside β-carbolines and 3 tryptophan-derived major natural product, psilocybin. Yet, N,N-dimethyl-l-tryptophan, are presented as well as current 4 recent research has revealed a more diverse secondary knowledge on their biosynthesis is provided. The multidiscipli- 5 metabolism that originates from this amino acid. In this nary character of natural product research is demonstrated, and 6 minireview, the focus is laid on l-tryptophan and the various pharmacological, medicinal, ecological, biochemical, and evolu- 7 Psilocybe natural products and their metabolic routes are tionary aspects are included. 8 9 1. Introduction In this minireview, we focus on the biosynthetic routes of L- 10 tryptophan-derived natural products in Psilocybe mushrooms. -

(12) United States Patent (10) Patent No.: US 9,687,864 B2 Fulton Et Al
USOO9687864B2 (12) United States Patent (10) Patent No.: US 9,687,864 B2 Fulton et al. (45) Date of Patent: Jun. 27, 2017 (54) SYSTEMAND METHOD FOR ENHANCED 428/25 (2015.01); Y10T 428/31504 (2015.04); ELECTROSTATIC DEPOSITION AND Y10T 428/31507 (2015.04); SURFACE COATINGS (Continued) (58) Field of Classification Search (71) Applicant: Battelle Memorial Institute, USPC .......... 118/620–640; 23.9/690 708; 427/458, Columbus, OH (US) 427/475 4.86 (72) Inventors: John L. Fulton, Richland, WA (US); See application file for complete search history. George S. Deverman, Richland, WA (US); Dean W. Matson, Kennewick, (56) References Cited CA (US); Clement R. Yonker, Kennewick, WA (US); C. Douglas U.S. PATENT DOCUMENTS Taylor, Franklinton, NC (US); James 3,087,860 A 4, 1963 Endicott B. McClain, Raleigh, NC (US); Joseph 3,123,077 A 3, 1964 Alcamo M. Crowley, Cambria, CA (US) (Continued) (73) Assignee: Battelle Memorial Institute, Columbus, OH (US) FOREIGN PATENT DOCUMENTS CA 2589761 12, 2004 (*) Notice: Subject to any disclaimer, the term of this CN 1465410 1, 2004 patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 0 days. (21) Appl. No.: 14/310,960 OTHER PUBLICATIONS Abreu Filho et al., “Influence of metal alloy and the profile of (22) Filed: Jun. 20, 2014 coronary stents in patients with multi-vessel coronary disease.” Clinics 66(6):985-989 (2011). (65) Prior Publication Data (Continued) US 2015/OO40827 A1 Feb. 12, 2015 Related U.S. Application Data Primary Examiner — Yewebdar Tadesse (74) Attorney, Agent, or Firm — Lerner, David, (62) Division of application No. -

Ergot Alkaloid Biosynthesis in Aspergillus Fumigatus : Association with Sporulation and Clustered Genes Common Among Ergot Fungi
Graduate Theses, Dissertations, and Problem Reports 2009 Ergot alkaloid biosynthesis in Aspergillus fumigatus : Association with sporulation and clustered genes common among ergot fungi Christine M. Coyle West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Coyle, Christine M., "Ergot alkaloid biosynthesis in Aspergillus fumigatus : Association with sporulation and clustered genes common among ergot fungi" (2009). Graduate Theses, Dissertations, and Problem Reports. 4453. https://researchrepository.wvu.edu/etd/4453 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Ergot alkaloid biosynthesis in Aspergillus fumigatus: Association with sporulation and clustered genes common among ergot fungi Christine M. Coyle Dissertation submitted to the Davis College of Agriculture, Forestry, and Consumer Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Genetics and Developmental Biology Daniel G. Panaccione, Ph.D., Chair Kenneth P. Blemings, Ph.D. Joseph B. -

United States Patent
Patented Aug. 17, 1948 2,447,214 UNITED STATES PATENT OFF ICE 2,447,214 OPTICALLY ACTIVE SALTS OF THE LY SERGIC AND SOLYSERGIC ACD DE RVATIVES AND A PROCESS FOR, THER PREPARATION AND SOLATION Arthur Stoll and Albert Hofmann, Basel, Switzer land, assignors to Sandoz Ltd., Fribourg, Swit zerland, a Swiss firm No Drawing. Application August 23, 1943, Serial No. 499,714. In Switzerland September 16, 1942 16 Claims. (CI. 260-236) 2 The preparation and the isolation of the thera The synthetically prepared derivatives of the peutically valuable and active derivatives con lysergic acid which also correspond to the above tained in ergot is a problem that has occupied cited formula possess also the same lability as chemistry and pharmacy for more than 120 years. the natural lysergic acid derivatives. Their is0 Actually it is known that the action of ergot is 5 lation and preparation encounters the same dif due to the alkaloids contained therein, which have ficulties as in the case of the lysergic acid hydra been isolated in recent years and which are zides C15H15N2CONHNH2 (made according to U. S. always present as pairs of isomers. Chrono Letters Patent 2,090,429) and in the case of the logically the following alkaloids have become alkaloids of the type of ergobasine, which can be 0 prepared by partial synthesis and in which the known up to noW: lysergic acid is combined with an amine in form Ergotinine (1875). Ergotoxine (1906) of an acid amide (see U. S. Letters Patent No. Ergotamine (1918) Ergotaminine (1918) 2,090,430). -

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water). -

Fused Tricyclic Dual Inhibitors of Cdk 4/6 and Flt3
(19) TZZ ¥¥_T (11) EP 2 937 349 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 28.10.2015 Bulletin 2015/44 C07D 471/14 (2006.01) C07D 519/00 (2006.01) A61K 31/519 (2006.01) A61P 35/00 (2006.01) (2006.01) (21) Application number: 15161337.9 A61P 35/02 (22) Date of filing: 21.03.2012 (84) Designated Contracting States: • Keegan, Kathleen S. AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Bainbridge Island, WA Washington 98110 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • LI, Zhihong PL PT RO RS SE SI SK SM TR Millbrae, CA California 94030 (US) Designated Extension States: • Lively, Sarah E. BA ME San Carlos, CA California 94070 (US) • McGee, Lawrence R. (30) Priority: 23.03.2011 US 201161466841 P Pacifica, CA California 94044 (US) •Ragains,Mark L. (62) Document number(s) of the earlier application(s) in Fort Worth, TX Texas 76109 (US) accordance with Art. 76 EPC: • Wang, Xianghong 12711738.0 / 2 688 887 Dublin, CA California 94568 (US) • Weidner, Margaret F. (71) Applicant: AMGEN INC. Woodinville, WA Washington 98072 (US) Thousand Oaks, CA 91320-1799 (US) • Zhang, Jian Foster City, CA California 94404 (US) (72) Inventors: • Chen, Xiaoqi (74) Representative: Hoffmann Eitle Palo Alto, CA California 94303 (US) Patent- und Rechtsanwälte PartmbB •Dai,Kang Arabellastraße 30 Albany, CA California 94706 (US) 81925 München (DE) • Duquette, Jason A. Millbrae, CA California 94030 (US) Remarks: • Gribble, Michael W., Jr. This application was filed on 12-05-2015 as a San Francisco, CA California 94110 (US) divisional application to the application mentioned • Huard, Justin N. -

Exploring New Pharmacology and Toxicological Screening and Safety
Zaman et al. BMC Complementary and Alternative Medicine (2015) 15:121 DOI 10.1186/s12906-015-0635-2 RESEARCH ARTICLE Open Access Exploring new pharmacology and toxicological screening and safety evaluation of one widely used formulation of Nidrakar Bati from South Asia region Afria Zaman1, Md Shamsuddin Sultan Khan2*, Lucky Akter1, Sharif Hossain Syeed3, Jakia Akter4, Abdullah Al Mamun5, Md Ershad Alam5, Md Ahsan Habib5 and Md Abdul Jalil5 Abstract Background: Nidrakar Bati (NKB) is an herbal remedy consisted with seven medicinal herbs widely used to cure Somnifacient (sleeping aid) in South Asia as Ayurvedic medicinal system. In the present study, pharmacological and toxicological effects of this medicine was investigated in mice to validate the safety and efficacy of the herb. Methods: Organic solvent extracts NKB were prepared using maceration method. Effect of extracts on the central nervous system was evaluated using hypnotic activity assay. Effect of the extracts on metabolic activity, assessing involvement of thyroid was conducted using hypoxia test. analgesic and anti-inflammatory activities were assessed in mice using acetic acid induced writhing, formalin induced paw edema, xylene induced ear edema assays. Anxiolytic activity was performed using plus maze, climbing out and forced swimming tests. Effect of the extracts on psychopharmacological effect was carried out using locomotor activity tests (open field, Hole-board and Hole-cross tests). Neuropharmacological effect of the extracts was performed using motor coordination (rotarod test). Toxicological potential of the extract was evaluated using gastro-intestinal activity (gastric emptying and gastrointestinal motility tests). Results: The studied formulation reduced the CNS stimulant effects dose independently. In the hypoxia test, only a dose of 100 mg/kg of NKB decreased the survival time. -

A Placebo Controlled Investigation of the Effects of Tryptophan Or Placebo on Subjective and Objective Measures of Fatigue
European Journal of Clinical Nutrition (1998) 52, 425±431 ß 1998 Stockton Press. All rights reserved 0954±3007/98 $12.00 http://www.stockton-press.co.uk/ejcn A placebo controlled investigation of the effects of tryptophan or placebo on subjective and objective measures of fatigue A Cunliffe, OA Obeid and J Powell-Tuck Department of Human Nutrition, St Bartholomew's and Royal London School of Medicine and Dentistry, Queen Mary and West®eld College, London E1 2AD Objective: To examine the effect of L-tryptophan administration on subjective and objective measures of fatigue in healthy volunteers. Subjects: Six healthy volunteers (4M:2F) were recruited from staff and students at the College. Setting: Department of Human Nutrition, St. Bartholomews and the Royal London School of Medicine and Dentistry. Design: Subjects were tested for central and peripheral fatigue using a visual analogue scale, ¯icker fusion frequency, grip strength, reaction time and wrist ergometry. In addition, plasma free tryptophan concentrations and Trp:LNAA ratio were determined. Measurements were made before, and at 1, 2, 3 and 4 h after drinking one of two test drinks. The drinks were of either caffeine free diet Coca-Cola (placebo) or caffeine free diet Coca- Cola plus L-tryptophan (30 mg/kg: active drink). Each of the six subjects was tested after placebo and active drink with a one week washout period between test days. Results: Subjective fatigue was signi®cantly increased following tryptophan compared to placebo (P < 0.002), and objective measures of central fatigue were signi®cantly increased by tryptophan compared to placebo (¯icker fusion frequency: P < 0.001; reaction time P < 0.001).