CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Operation Theatre Technician Anurag
OPERATION THEATRE TECHNICIAN ANURAG. A. S ADARSH. A. S ANU BHAVAN VIDYA VIHAR, VEERALAM VALIYAPARAMBU ATTINGAL PO MARAYAMUTTOM PO TRIVANDRUM 695101 TRIVANDRUM 695124 Roll No. 1001 Roll No. 1008 AJULAL. T ANURAJ. S. R LINUBHAVANAM, THAZHAM VARUVILA PUTHEN VEEDU EAST KALLADA PO, MAMKOOTTAM THIRUPURAM PO KOLLAM, 691502 TRIVANDRUM 695133 Roll No. 1002 Roll No. 1009 AMAL NATH. R. M ANUSREE. A SARALAMANDIRAM SREELAKAM MADATHUVATHUKKAL KAROOR, POTHENCODE PO MITHIRMALA PO‐ 695610 TRIVANDRUM 695584 Roll No. 1003 Roll No. 1010 ANILA. P. V ARCHANA. S PUTHIYA VEETTIL ARCHANA BHAVAN POOKODE PO PULLOORKONAM, VIZHINJAM PO KANNUR 670691 TRIVANDRUM. 695521 Roll No. 1004 Roll No. 1011 ANTO VINCENT. T ARCHANA. S ANTO NIVAS SREEPADMAM, LMSRA‐65 VIRALY, UCHAKKADA PO L. M. S. JUNCTION, ATTINGAL NEYYATTINKARA TRIVANDRUM, 695101 TRIVANDRUM,695506 Roll No. 1012 Roll No. 1005 ARUN. G. J ANUJA. S. S AMBADI, VAMANAPURAM ADITHYA BHAVAN VAMANAPURAM PO KATTACHAL KUZHI PO TRIVANDRUM, 695606 BALARAMAPURAM Roll No. 1013 TRIVANDRUM 695501 Roll No. 1006 ASWANI. R LAKSHMIGOVINDAM' ANURADHA. S. P KAYAKKAL HOUSE, PAYAMBRA POST SAFALYA KUNNAMANGALAM (VIA) PUTHENVEEDU KOZHIKODE 673571 PALLIPPURAM PO Roll No. 1014 TRIVANDRUM 695316 Roll No. 1007 ASWATHI. S ILANGO. K KRISHNA VILASOM NO. 18, K. K. ILLAM KADATHOOR, K. S. PURAM PO KALIAMMAN KOVIL STREET KARUNAGAPPALLY KEEZHAKASAKUDY, KOTTUCHERRY KOLLAM 690544 KARAIKAL 609609 Roll No. 1015 Roll No. 1023 BABU. T. N JEENA. J. V NO. 20, CONTRACTOR SUBRAMANI FIRST STREET THEKKEYIDA VILAKATHU THORAPADI, VELLORE MELE PUTHEN VEEDU TAMIL NADU, 632002 AMOTTUKONAM, CHAIKOTTUKONAM PO Roll No. 1016 TRIVANDRUM 695122 Roll No. 1024 BIFIN. B ARUMALOORKONAM JINI. M. G MEKKE PLANKALA VEEDU SOUPARNIKA KUNNATHUKAL, KARAKONAM PO MAMPALLIKUNNAM TRIVANDRUM 695504 CHATHANNUR PO Roll No. -

Payment Locations - Muthoot
Payment Locations - Muthoot District Region Br.Code Branch Name Branch Address Branch Town Name Postel Code Branch Contact Number Royale Arcade Building, Kochalummoodu, ALLEPPEY KOZHENCHERY 4365 Kochalummoodu Mavelikkara 690570 +91-479-2358277 Kallimel P.O, Mavelikkara, Alappuzha District S. Devi building, kizhakkenada, puliyoor p.o, ALLEPPEY THIRUVALLA 4180 PULIYOOR chenganur, alappuzha dist, pin – 689510, CHENGANUR 689510 0479-2464433 kerala Kizhakkethalekal Building, Opp.Malankkara CHENGANNUR - ALLEPPEY THIRUVALLA 3777 Catholic Church, Mc Road,Chengannur, CHENGANNUR - HOSPITAL ROAD 689121 0479-2457077 HOSPITAL ROAD Alleppey Dist, Pin Code - 689121 Muthoot Finance Ltd, Akeril Puthenparambil ALLEPPEY THIRUVALLA 2672 MELPADAM MELPADAM 689627 479-2318545 Building ;Melpadam;Pincode- 689627 Kochumadam Building,Near Ksrtc Bus Stand, ALLEPPEY THIRUVALLA 2219 MAVELIKARA KSRTC MAVELIKARA KSRTC 689101 0469-2342656 Mavelikara-6890101 Thattarethu Buldg,Karakkad P.O,Chengannur, ALLEPPEY THIRUVALLA 1837 KARAKKAD KARAKKAD 689504 0479-2422687 Pin-689504 Kalluvilayil Bulg, Ennakkad P.O Alleppy,Pin- ALLEPPEY THIRUVALLA 1481 ENNAKKAD ENNAKKAD 689624 0479-2466886 689624 Himagiri Complex,Kallumala,Thekke Junction, ALLEPPEY THIRUVALLA 1228 KALLUMALA KALLUMALA 690101 0479-2344449 Mavelikkara-690101 CHERUKOLE Anugraha Complex, Near Subhananda ALLEPPEY THIRUVALLA 846 CHERUKOLE MAVELIKARA 690104 04793295897 MAVELIKARA Ashramam, Cherukole,Mavelikara, 690104 Oondamparampil O V Chacko Memorial ALLEPPEY THIRUVALLA 668 THIRUVANVANDOOR THIRUVANVANDOOR 689109 0479-2429349 -

NATIONAL MEANS CUM MERIT SCHOLARSHIP EXAMINATION (NMMSE)-2019 (FINAL LIST of ELIGIBLE CANDIDATES) THIRUVANANTHAPURAM DISTRICT GENERAL CATEGORY Sl
NATIONAL MEANS CUM MERIT SCHOLARSHIP EXAMINATION (NMMSE)-2019 (FINAL LIST OF ELIGIBLE CANDIDATES) THIRUVANANTHAPURAM DISTRICT GENERAL CATEGORY Sl. Caste ROLL NO Applicant Name School_Name No Category 1 42192790174 SREEHARI VINOD General Govt. Model HSS For Boys Attingal , Attingal 2 42192830290 GOPIKA I G General Govt. V.H.S.S. Kallara , KALLARA 3 42192730328 ARATHY M General GOVT. H S S, NEDUVELI, KONCHIRA, VEMBAYAM , 4 42192750125 ANAND SWAROOP J S General Govt. H S S Elampa , Elampa 5 42192740003 AMAL A L General L. V. H. S. Pothencode , Pothencode 6 42192860293 DEVANARAYANAN S R General P. P. M. H. S. Karakonam , karakonam 7 42192810350 KRIPA SUDISH General R R V GHSS Kilimanoor , kilimanoor 8 42192830280 ASNA S General Govt. V.H.S.S. Kallara , KALLARA 9 42192870029 AKHILA S General St. Thomas H. S. S. Amboori , Amboori 10 42192830299 MIDHUNA S NAIR General Govt. V.H.S.S. Kallara , KALLARA 11 42192740032 RESHMA S R General St. Goretti's Girls H. S. S. Nalanchira , Nalanchira 12 42192760120 ASHTAMI A S General DBHS Vamanapuram , vamanapuram 13 42192790241 GANGA G PRASANNAN General Govt H S S For Girls Attingal , Attingal 14 42192790227 ATHIDI ANILKUMAR General Govt H S S For Girls Attingal , Attingal 15 42192810135 DEVIKA B General Govt. HSS Kilimanoor , kilimanoor 16 42192820005 ADWAITH S R General Govt V H S S Njekkad , NJEKKAD 17 42192830371 RISHAV RAJ General M R M K M M H S S Edava , EDAVA 18 42192850125 SHANU S General St. Mary`s H. S. S. Vizhinjam , Vizhinjam 19 42192840321 JAYASREE J S General New H. S. -

Reg No: Name Reg.No Reg Date 455188 MONICA CHRISTOPHER 77001 14--07--2020 412394 ARYA.V 77003 14--07--2020 328998 Aswathysasidha
List - XXII Permanent Registration Number alone Allotted (Continuation from List XXI) Details of Permanent Register number allotted to Modern medicine Doctors (Applications received up to 20/08/2020) Certificate will be issued after completing all formal procedures Reg No: Name Name of College` Reg.No Reg Date 455188 MONICA CHRISTOPHER TRAVANCORE MEDICAL COLLEGE, MEDICITY, KOLLAM 77001 14--07--2020 412394 ARYA.V JUBILEE MISSION MEDICAL COLLEGE & RESEARCH INSTITUTE, THRISSUR 77003 14--07--2020 328998 AswathySasidharan Government Medical College, Thrissur 77005 14--07--2020 336170 Anjumol T.A Government Medical College, Kottayam 77006 14--07--2020 463840 SHAMSHEEDA GOVERNMENT MEDICAL COLLEGE , KANNUR 77751 18--08--2020 512297 AKSHAY M DILEEP Government Medical College, Kottayam 77752 18--08--2020 506767 NOUREEN FAZAL Government Medical College, Thrissur 77753 18--08--2020 508435 FIDHA FATHIMA SADAR Government Medical College, Kottayam 77754 18--08--2020 505802 NAHJA ROSHIN A.M. TRAVANCORE MEDICAL COLLEGE, MEDICITY, KOLLAM 77756 18--08--2020 481395 AISWARYA VIJAY V J SREE NARAYANA INSTITUTE OF MEDICAL SCIENCES, ERNAKULAM 77757 18--08--2020 506776 SONA MARIA PETER PK DAS INSTITUTE OF MEDICAL SCIENCES, PALAKKAD 77758 18--08--2020 445548 SWABEEHA ABOOBACKER PK DAS INSTITUTE OF MEDICAL SCIENCES, PALAKKAD 77759 18--08--2020 507536 DHEERAJ T. K. D.M. Wayanad Institute of Medical Sciences, Wayanad 77760 18--08--2020 499438 MURSHIDA P MALABAR MEDICAL COLLEGE HOSPITAL AND RESEARCH CENTER, KOZHIKODE 77761 18--08--2020 383612 Bini Cherian JUBILEE MISSION -
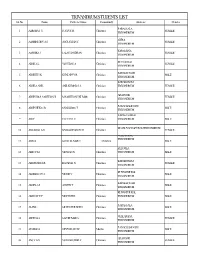
Thiruvananthapuram
TRIVANDRUM STUDENTS LIST SL No Name Father's Name Community Address Gender PARASSALA 1 AARSHA J U JUSTUS H Christian FEMALE TRIVANDRUM AYIRA 2 AASBINI DEV A S AUGUSTIAN C Christian FEMALE TRIVANDRUM KARAMANA 3 AASHIKA J JALACHANDRAN Christian FEMALE TRIVANDRUM KOTTACKAL 4 ABHIJA L YESUDAS A Christian FEMALE TRIVANDRUM KARAKKONAM 5 ABHIJITH K KUNJAPPY R Christian MALE TRIVANDRUM KARAKONAM 6 ABHILA ANIL ANIL KUMAR S S Christian TRIVANDRUM FEMALE ARAYOOR 7 ABHISHA S SANTHOSH M SANTHOSH KUMAR Christian FEMALE TRIVANDRUM PANACHAMOODU 8 ABHISHEK G B GNANADAS T Christian MALE TRIVANDRUM PARASUVAIKAL 9 ABI C CLEETUS C Christian TRIVANDRUM MALE DHANUVACHAPURAM TRIVANDRUM 10 ABIJADAS A G GNANADHASON D Christian FEMALE VELLARADA, TRIVANDRUM 11 ABIN S SUNIL KUMAR C Christian MALE ELLUVILA 12 ABIN V M VIJAYAN N Christian TRIVANDRUM MALE KARAKONAM 13 AISHWARYA R RAJADAS N Christian FEMALE TRIVANDRUM KUNNATHUKAL 14 AKHILESH V A VINOD Y Christian MALE TRIVANDRUM KARAKKONAM 15 AKHIN A S AUSTIN T Christian MALE TRIVANDRUM KUNNATHUKAL 16 AKSHAY V P VINCENT R Christian TRIVANDRUM MALE PARASSALA 17 ALAN L SILVESTER LEEN Christian MALE TRIVANDRUM VELLARADA, 18 ALEENA S SAJI KUMAR S Christian FEMALE TRIVANDRUM PANACHAMOODU 19 ALSHAD.S SEYYAD ALI M Muslim MALE TRIVANDRUM ARAYOOR 20 ANCY A V VIJAYAKUMAR P Christian FEMALE TRIVANDRUM TRIVANDRUM STUDENTS LIST SL No Name Father's Name Community Address Gender ELLUVILA 21 ANCY MARY V S VINCENT M Christian FEMALE TRIVANDRUM VELLARADA 22 ANEENA V J VIJAYAKUMAR K Christian FEMALE TRIVANDRUM KOOTHALI 23 ANEESHA V LOWRANCE -

Voluntary Organizations.Pdf
1 List of voluntary organization with location of centre implementing Rajiv Gandhi National Creche Scheme - Kerala (Decentralized) Sl.N Name of the voluntary Full address of No. of units o. organization with full location of the centre sanctioned address 1 2 3 4 THIRUVANANTHAPURAM 1. F.No.CR-1/TVPM/2012-13/ R.C.Church 1 464 dt.08.08.2012 Edanji, Malayilkada, Manchavilakom.P.O, Anavoor Mahila Samajam Neyyattinkara Taluk, Kollayil Panchyath Anavoor, Perumkadavila, Thiruvananthapuram, Thiruvananthapuram 2. F.No.CR-2/TVPM/2012-13/ Aralummoodu, 1 464 dt. 08.08.2012 Trivandrum Dt. Aralummodu Vanitha Kshema Kendram, Aralummodu, Thiruvananthapuram. 3. F.No.CR-4/TVPM/2012-13/ 1. St.Joseph’s Church, 4 464 dt. 08.08.2012 Paliyode, Kottackal.P.O, Trivandrum. Baptist Memorial Mahila 2. St.Joseph’s Church, 2 Samajam, Paliyode, Kottackal.P.O, Trivandrum. Kottackal P.O., Perumkadavila, Thiruvananthapuram. 3. Holy Family Church, Chamavila, Kudayal.P.O, Trivandrum. 4. St.Mary’s Church, Thannikuzhy, Trivandrum. 3 4 F.No.CR-5/ TVPM/2012-13/ 1.Pulininnakattampotta, 464 dt. 08.08.2012 Kuttavila, Paraniyam, Poovar.P.O, Trivandrum. Bharath Social Service Centre, 7 Paraniyam, Poovar P.O. 2.Opp: to Church, Baby Thiruvananthapuram Creche, Karikkottakary, Kannur. 3.Near Milk Co- operative Society, Edapuzha, Iritty Block, Kannur. 4.BSSC Creche unit, Keezhpally, Kannur 5.Veerpad, Aralam Panchayath, Iritty Block, Kannur. 6.Madarsa L.P.School Aralam, Aralam Panchayath, Kannur. 7.Payam East, Iritty Block, Kannur. 5. F.No.CR-6/ TVPM/2012-13/ Near Milk Society, 1 464 dt. 08.08.2012 Kottukonam Road , 4 Velatharakonam, Bharatha Yathra Centre, Elluvila P.O. -
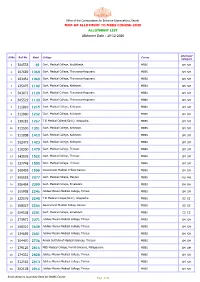
ALLOTMENT LIST MOP-UP ALLOTMENT to MBBS COURSE-2020 Allotment Date
Office of the Commissioner for Entrance Examinations, Kerala MOP-UP ALLOTMENT TO MBBS COURSE-2020 ALLOTMENT LIST Allotment Date : 20-12-2020 Allotment Sl.No. Roll No. Rank College Course Category 1 304533 39 Govt. Medical College, Kozhikkode. MBBS SM SM 2 107669 1068 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 3 103451 1069 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 4 135075 1102 Govt. Medical College, Kottayam. MBBS SM SM 5 303073 1128 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 6 305529 1139 Govt. Medical College, Thiruvananthapuram. MBBS SM SM 7 111810 1215 Govt. Medical College, Kottayam. MBBS SM SM 8 312980 1232 Govt. Medical College, Kottayam. MBBS SM SM 9 130281 1267 T D Medical College(Govt.), Alappuzha. MBBS SM SM 10 311530 1351 Govt. Medical College, Kottayam. MBBS SM SM 11 311808 1410 Govt. Medical College, Kottayam. MBBS SM SM 12 302475 1423 Govt. Medical College, Kottayam. MBBS SM SM 13 110300 1479 Govt. Medical College, Thrissur. MBBS SM SM 14 143076 1522 Govt. Medical College, Thrissur. MBBS SM SM 15 137748 1590 Govt. Medical College, Thrissur. MBBS SM SM 16 300439 1599 Government Medical College Kannur MBBS SM SM 17 305518 2077 Govt. Medical College, Manjeri. MBBS MU MU 18 206454 2099 Govt. Medical College, Ernakulam MBBS BH BH 19 105958 2246 Jubilee Mission Medical College, Thrisur. MBBS SM SM 20 122576 2248 T D Medical College(Govt.), Alappuzha. MBBS EZ EZ 21 168027 2266 Government Medical College Kannur MBBS EZ EZ 22 304538 2281 Govt. Medical College, Ernakulam MBBS EZ EZ 23 173971 2371 Jubilee Mission Medical College, Thrisur. -

Accused Persons Arrested in Kollam Rural District from 02.02.2020To08.02.2020
Accused Persons arrested in Kollam Rural district from 02.02.2020to08.02.2020 Name of Name of Name of the Place at Date & Arresting the Court Sl. Name of the Age & Cr. No & Police father of Address of Accused which Time of Officer, at which No. Accused Sex Sec of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 PANAVILA THEKKATHIL 144/2020 EAST 08-02-2020 30, VEEDU, MOONNUM U/s 279 IPC KALLADA BAILED BY 1 UNNI.P POOMANI at 23:05 ARUN Male PERUMPUZHA UKKU &185 OF MV (Kollam POLICE Hrs MURRY, ACT Rural) ILAMBALLOOR CHATTAMULA 234/2020 08-02-2020 PUNALOO 44, TEKKAI U/s 279 IPC BAILED BY 2 SANTHOSH KUNNAN T.B.JN at 23:00 R (Kollam RAJEEV.J Male VEETIL,KEZHANI AND 185 OF POLICE Hrs Rural) YIL, ATTINGAL MV ACT 245/2020 PATHANA PARATHUNDIL 08-02-2020 SI RAGHAVA 28, KALLUM U/s 279 IPC PURAM BAILED BY 3 RATHEESH VEEDU MALOOR at 22:23 PUSHPAKU N Male KADAVU & 185 MV (Kollam POLICE PATHANAPURAM Hrs MAR ACT Rural) 194/2020 Krishna Vilasam, 08-02-2020 KADAKKA Krishnadas 37, U/s 279 IPC Saju V, SI of BAILED BY 4 Jayaram Irakkuzhi, Market Jn at 20:40 L (Kollam Nair Male & Sec 185 of Police POLICE Mangodu Village Hrs Rural) MV ACt 244/2020 PATHANA VRINDHAVANAM 08-02-2020 SI 35, KALLUM U/s 279 IPC PURAM BAILED BY 5 ARUN ASHOKAN KARAVALOOR at 22:15 PUSHPAKU Male KADAVU & 185 MV (Kollam POLICE PUNALUR Hrs MAR ACT Rural) AMPILI 243/2020 PATHANA BHAVANAM 08-02-2020 SI RAMAKRIS 38, KALLUM U/s 279 IPC PURAM BAILED BY 6 ANEESH. -

House Details
House Details Sl Localbody Name Ward Door Sub Owner Name Address Mobile No Type of Damage No No No No Damage Percentage 1 Amboori Grama 2 3 Temp Sudha Vinod Thadatharikathu Veedu , kurishumala 9526956232 Partial >75% Damage Panchayat damage for Buildings 2 Amboori Grama 10 136 Santhosh Ayyaramkuzhi Veedu , Kuttamala 8129565517 Partial >75% Damage Panchayat damage for Buildings 3 Amboori Grama 12 30 Samkutty S Kyzhivila Rodarikathu veedu , Kamdamthitta , 9846784961 Partial >75% Damage Panchayat Mayam P.O damage for Buildings 4 Attingal Municipality 13 78 Sheeja. S Thekkavila Veedu, Near polytechnic, 9562343868 Partial >75% Damage Valiyakunnu damage for Buildings 5 Chemmaruthy Grama 2 700 Manoj S Charuvila veedu, Kovoor, Palayalamkunnu PO. 8943040493 Partial >75% Damage Panchayat damage for Buildings 6 Chemmaruthy Grama 8 425 Suseela Kollam Vilakam Chemmaruthy Vadaseerikonam 7025580518 Partial >75% Damage Panchayat PO damage for Buildings 7 Kadakkavoor Grama 10 240 Vimaladevi Sandhoshbhavan, kadakkavoor 9048260524 Partial >75% Damage Panchayat damage for Buildings 8 Kadakkavoor Grama 11 2 A Vasanthi Vayalilthitta, kochupalam, kadakkavoor 9567020013 Partial >75% Damage Panchayat damage for Buildings 9 Karavaram Grama 1 611 Sudharsanan Meenakshi mandiram karavaram, pavalla, 7025531063 Partial >75% Damage Panchayat R kallambalam po,karavaram thiruvananthapuram, damage for kerala-695605 Buildings 10 Karavaram Grama 10 103 Ravindran M Preetha pulari kannelikonam vanchiyoor p.o 9895854483 Partial >75% Damage Panchayat alamcode damage for Buildings -
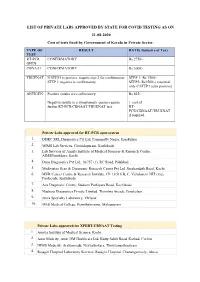
LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 21-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 21-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Private Labs approved for RT-PCR open system 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam 2. MIMS Lab Services, Govindapuram, Kozhikode 3. Lab Services of Amrita Institute of Medical Sciences & Research Centre, AIMSPonekkara, Kochi 4. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad 5. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Sreekandath Road, Kochi 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode 8. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam 9. Jeeva Specialty Laboratory, Thrissur 10. MES Medical College, Perinthalmanna, Malappuram Private Labs approved for XPERT/CBNAAT Testing 1. Amrita Institute of Medical Science, Kochi 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin 3. NIMS Medicity, Aralumoodu, Neyyattinkara, Thiruvananthapuram 4. Rajagiri Hospital Laboratory Services, Rajagiri Hospital, Chunangamvely, Aluva 5. Micro Health LAbs, MPS Tower, Kozhikode 6. Believers Church Medical College Laboratory, St Thomas Nagar, Kuttapuzha P.O., Thiruvalla 7. -

Change Name.Pdf
758 KERALA GAZETTE [PART IV NOTIFICATION NOTIFICATION It is hereby notified for the information of all It is hereby notified for the information of all authorities concerned and the public that I, Aswathy. S, authorities concerned and the public that I, John. E, Elanjikunnuvila Veedu, Punnakulam, Kottukal P. O., Neyyattinkara Taluk, Thiruvananthapuram District, J. S. A. Nivas, Nulliyode, Olathanni, Thirupuram P. O., Pin-695 501, holder of S. S. L. C. No. Q 176991 with Neyyattinkara Taluk, Thiruvananthapuram District, Register No. 134032 of March 2015, have embraced Pin-695 133, holder of Extract of School Admission Christianity from Hindu, Sambavar Community as per Register with Admission No. 559, date of Admission Baptism Certificate dated 1-10-2017, issued from India 21-5-1969 issued from Victory Vocational Higher Evangelical Lutheran Church, Balaramapuram, Secondary School, Olathanni, Neyyattinkara; Ration Card Thiruvananthapuram with the same name. Hereafter I will be a member of Christian, Sambavar Community. No. 1106018921 (Sl. No. 4), dated 15-3-2017, issued by the Taluk Supply Officer, Neyyattinkara, also known as This change will come into effect in all records related to me. Enose John in the Aadhaar No. xxxxxxxxxxxxx , issued from Unique Identification Authority of India; Electoral Kottukal, Identity Card No. BRP1312313, dated 3-7-2015, issued by 25-11-2017. ASWATHY. S the Electoral Registration Officer, Neyyattinkara Assembly NOTIFICATION Constituency, is one and the same person. Hereafter I will be known by the name Enose John only and will sign It is hereby notified for the information all authorities accordingly. concerned and public that I, Christal Jayaraj C, Alampara Vadakkethattu Puthen Veedu, Parasuvaikkal P. -

DIOCESE of NEYYATTINKARA His Excellency Rt. Rev. Dr. Vincent Samuel S.T.D. Bishop of Neyyattinkara Bishop's House, Neyyattinka
DIOCESE OF NEYYATTINKARA His Excellency Rt. Rev. Dr. Vincent Samuel S.T.D. Bishop of Neyyattinkara Bishop’s House, Neyyattinkara, Aralummood P.O. Trivandrum – 695 123, Kerala, India : 0471-2223133, 2220693, Fax: 0471-2222262 :[email protected] : [email protected] Web : www.neyyattinkaradiocese.org B. 10.8.1950 / O. 19.12.1975 / E.O. 01.11.1996 F. 27th September 1. Very. Rev. Msgr. G. Christudas Vicar General Bishop’s House, Neyyattinkara Aralummood P.O. Trivandrum – 695 123 : 0471-2227137 (P), 2223133, 2220693 Mob. 9847069309,9447534132 : [email protected] [email protected] Manager Emmanuel College of Arts and Science &Emmaneul College of BEd. Training Vazhichal, Kudappanamood P.O Thiruvanathapuram : 0471-2248416, 2248113 : [email protected] [email protected] : www.emmanuelcollege.ac.in B. 14.01.1950 / O. 19.12.1975 / F. Christ the King 2. Very Rev. Msgr. D. Selvarajan JCD Episcopal Vicar &Regional Coordinator (Neyyattinkara Region) St. Xavier’s Church, Thirupuram Thirupuram P.O., Trivandrum - 695 133 Mob. 9447864433 & Director, Logos Pastoral Centre San Jose Nagar Neyyattinkara, Trivandrum – 695 121 : 0471-2221194 : [email protected] &. Judicial Vicar Bishop’s House, Neyyattinkara Aralummood P.O., Trivandrum – 695 123 : 0471- 2221941, 0471- 2222760 B. 27.01.1962 / O. 23.12.1987 / F. Christ the King 3. Very. Rev. Msgr. V.P. Jose Episcopal Vicar &Coordinator of Ministries Logos Pastoral Center, Neyyattinkara, San Jose Nagar 695121 &. P.P.,Our Lady of Assumption Forane Church Vlathankara P.O., Trivandrum – 695 134 : 0471-2236165, C. 2236617, Mob. 08547678999, 8921922356 [email protected] [email protected] B. 19.05.1967/ O. 12.04.1994 / F.19th March 4.