Food Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States July 2016 2 Table of Contents
Deuterium Labelled Compounds United States July 2016 2 Table of Contents International Distributors 3 Corporate Overview 4 General Information 5 Pricing and Payment 5 Quotations 5 Custom Synthesis 5 Shipping 5 Quality Control 6 Quotations 6 Custom Synthesis 6 Shipping 6 Quality Control 6 Chemical Abstract Service Numbers 6 Handling Hazardous Compounds 6 Our Products are Not Intended for Use in Humans 7 Limited Warranty 7 Packaging Information 7 Alphabetical Listings 8 Stock Clearance 236 Products by Category 242 n-Alkanes 243 α-Amino Acids, N-Acyl α-Amino Acids, N-t-BOC Protected α-Amino Acid 243 and N-FMOC Protected α-Amino Acids Buffers and Reagents for NMR Studies 245 Detergents 245 Environmental Standards 246 Fatty Acids and Fatty Acid Esters 249 Flavours and Fragrances 250 Gases 253 Medical Research Products 254 Nucleic Acid Bases and Nucleosides 255 Pesticides and Pesticide Metabolites 256 Pharmaceutical Standards 257 Polyaromatic Hydrocarbons (PAHs), Alkyl-PAHs, Amino-PAHs, 260 Hydroxy-PAHs and Nitro-PAHs Polychlorinated Biphenyls (PCBs) 260 Spin Labels 261 Steroids 261 3 International Distributors C Beijng Zhenxiang H EQ Laboratories GmbH Australia K Technology Company Graf-von-Seyssel-Str. 10 Rm. 15A01, Changyin Bld. 86199 Augsburg Austria H No. 88, YongDingLu Rd. Germany Beijing 100039 Tel.: (49) 821 71058246 Belgium J China Fax: (49) 821 71058247 Tel.: (86) 10-58896805 [email protected] China C Fax: (86) 10-58896158 www.eqlabs.de Czech Republic H [email protected] Germany, Austria, China Czech Republic, Greece, Denmark I Hungary, -

Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S
foods Article Effects of Simultaneous Co-Fermentation of Five Indigenous Non-Saccharomyces Strains with S. cerevisiae on Vidal Icewine Aroma Quality Qian Ge 1,2,3, Chunfeng Guo 1,3, Jing Zhang 2, Yue Yan 2, Danqing Zhao 2, Caihong Li 2, Xiangyu Sun 1, Tingting Ma 1, Tianli Yue 1,3,4 and Yahong Yuan 1,3,4,* 1 College of Food Science and Engineering, Northwest A&F University, Yangling 712100, China; [email protected] (Q.G.); [email protected] (C.G.); [email protected] (X.S.); [email protected] (T.M.); [email protected] (T.Y.) 2 Institute of Quality Standard and Testing Technology for Agro-Products of Ningxia, Yinchuan 750002, China; [email protected] (J.Z.); [email protected] (Y.Y.); [email protected] (D.Z.); [email protected] (C.L.) 3 National Engineering Research Center of Agriculture Integration Test (Yangling), Yangling 712100, China 4 College of Food Science and Technology, Northwest University, Xi’an 710069, China * Correspondence: [email protected]; Tel./Fax: +86-029-87092261 Abstract: In this study, Vidal grape must was fermented using commercial Saccharomyces cerevisiae F33 in pure culture as a control and in mixed culture with five indigenous non-Saccharomyces yeast strains (Hanseniaspora uvarum QTX22, Saccharomycopsis crataegensis YC30, Pichia kluyveri HSP14, Metschnikowia pulcherrima YC12, and Rhodosporidiobolus lusitaniae QTX15) through simultaneous fermentation in a 1:1 ratio. Simultaneous fermentation inhibited the growth of S. cerevisiae F33 and Citation: Ge, Q.; Guo, C.; Zhang, J.; delayed the time to reach the maximum biomass. Compared with pure fermentation, the contents Yan, Y.; Zhao, D.; Li, C.; Sun, X.; Ma, of polyphenols, acetic esters, ethyl esters, other esters, and terpenes were increased by R. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -
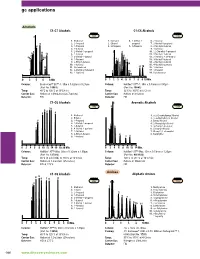
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Borneol - Camphor - Isoborneol
EXP. 35 A & B OXIDATION-REDUCTION SCHEME: BORNEOL - CAMPHOR - ISOBORNEOL LEARNING OBJECTIVES: To illustrate the concepts of oxidation and reduction in organic chemistry, to illustrate the stereochemical effects of these reactions in certain systems, to use IR spectroscopy to characterize diastereomers and monitor reactions. QUIZ PREPARATION: Recitation notes and readings assigned in the syllabus. OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY The concepts of oxidation and reduction in chemistry are fundamentally related to the loss and gain of electrons, respectively. For a specific atom, oxidation brings about an increase in the oxidation number, whereas reduction does the opposite. This is of course true in organic reactions as well, but it is frequently less clear where the gain or loss of electrons is taking place. For this reason, the concepts of oxidation and reduction are addressed from a different perspective in organic chemistry. Since carbon is the most important element in organic chemistry, we can define oxidation as a process whereby carbon gains bonds to more electronegative atoms. These can be any atoms more electronegative than carbon, such as chlorine or sulphur, but the most commonly seen in organic oxidations is oxygen. The more bonds to oxygen, the higher the oxidation state of the carbon involved. Examples of oxidation reactions are those that convert alkanes into alcohols, or alcohols into carbonyl compounds. The functional groups that contain the most highly oxidized form of carbon in organic compounds are the carboxylic acids and their derivatives. Reduction is the opposite of oxidation. We can define reduction as a process whereby carbon adds bonds to less electronegative atoms. -

Visible-Light-Driven Photooxidation of Alcohols Using Surface-Doped Graphitic Carbon Nitride
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is © The Royal Society of Chemistry 2017 Visible-Light-Driven Photooxidation of alcohols using surface-doped graphitic carbon nitride Wuyuan Zhang, Anna Bariotaki, Ioulia Smonou, and Frank Hollmann* Material and Methods Materials Unless stated otherwise all chemicals were purchased from Sigma-Aldrich, Fluka, Acros or Alfa-Aesar with the highest purity available and used without further treatment. Synthesis of g-C3N4 and carbon nanodots doped (CD-C3N4) The g-C3N4 was synthesized via the simple calcination (Carbolite furnace; CWF 12/5, 2400 W) of urea (10.0 g) at 600 °C for 4 h (5 °C/min), a yellowish powder was obtained after the cooling to room temperature.1 The carbon nanodots were synthesized via the thermal decomposition of sucrose with slight modification.2 Briefly, 0.75 g of sucrose was dissolved in 30 mL of MilliQ water and stirred at room temperature for 0.5 h. The solution was transferred into a 45 mL Teflon-lined stainless steel autoclave reactor, and heated at 180 °C for 5 h (10 °C/min). After cooling the reactor to room temperature, a brown mixture was obtained. The mixture was centrifuged (8000 rpm for 20 min), and the pellet was washed with water (3×) and freeze-dried. 3 In order to deposit carbon nanodots to the surface of g-C3N4, Liu et al’s procedures were adopted. Firstly, a stock solution of carbon nanodots (1 mg in 25mL water) was prepared. 15.0 mL of this stock solution was mixed with 15.0 mL of NH4OH (28 %) and sealed in a 45 mL Teflon-lined autoclave reactor. -

(12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry Et Al
US00757617OB2 (12) United States Patent (10) Patent No.: US 7,576,170 B2 Perry et al. (45) Date of Patent: Aug. 18, 2009 (54) CYCLIC SILOXANE COMPOSITIONS FOR 6,046,156 A 4/2000 Perry THE RELEASE OF ACTIVE INGREDIENTS 6,054,547 A 4/2000 Perry et al. 6,063,365 A 5, 2000 Schefer et al. (75) Inventors: Robert J. Perry, Niskayuna, NY (US); 6,075,111 A 6/2000 Perry et al. Mark D. Leatherman, Elmsford, NY 6,077.923 A 6/2000 Perry et al. (US); Shahid Murtuza, Albany, NY 6,083,901 A 7/2000 Perry et al. (US) 6,121,343 A 9/2000 Hongo et al. 6,143,309 A 11/2000 Legrow et al. (73) Assignee: Momentive Performance Materials, 6,153,578 A 11/2000 Perry Albany, NY (US) 6,200,949 B1 3/2001 Reijmer et al. 6,228,380 B1 5, 2001 LeGrow et al. (*) Notice: Subject to any disclaimer, the term of this 6,262,287 B1 7/2001 Anderson et al. patent is extended or adjusted under 35 6,267,977 B1 7/2001 LeGrow et al. U.S.C. 154(b) by 993 days. 6,309,715 B1 10/2001 Lindauer et al. 6,322,777 B1 1 1/2001 Perry et al. (21) Appl. No.: 10/742,415 6,325,274 B2 12/2001 Esumi et al. 6,325,859 B1 12/2001 De Roos et al. (22) Filed: Dec. 19, 2003 6,435,423 B2 8/2002 Hurry et al. (65) Prior Publication Data 6,624,136 B2 9, 2003 Guerinet al. -

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -

PRODUCTION of ETHYL LEVULINATE VIA ESTERIFICATION REACTION of LEVULINIC ACID in the PRESENCE of Zro2 BASED CATALYST
Malaysian Journal of Analytical Sciences, Vol 23 No 1 (2019): 45 - 51 DOI: https://doi.org/10.17576/mjas-2019-2301-06 MALAYSIAN JOURNAL OF ANALYTICAL SCIENCES ISSN 1394 - 2506 Published by The Malaysian Analytical Sciences Society PRODUCTION OF ETHYL LEVULINATE VIA ESTERIFICATION REACTION OF LEVULINIC ACID IN THE PRESENCE OF ZrO2 BASED CATALYST (Penghasilan Etil Levulinat Melalui Pengesteran Asid Levulinik dengan Kehadiran Mangkin Berasaskan ZrO2) Dorairaaj Sivasubramaniam1, Nor Aishah Saidina Amin1*, Khairuddin Ahmad1, Nur Aainaa Syahirah Ramli2 1Chemical Reaction Engineering Group (CREG), Faculty of Chemical Engineering, Universiti Teknologi Malaysia, 81300 Skudai, Johor, Malaysia 2Advanced Oleochemical Technology Division, Malaysian Palm Oil Board (MPOB), 6, Persiaran Institusi, Bandar Baru Bangi, 43000 Kajang, Selangor, Malaysia *Corresponding author: [email protected] Received: 13 April 2017; Accepted: 17 April 2018 Abstract Ethyl levulinate is widely used as a fuel additive, flavor or fragrance and as a component of fuel blending. This study focused on the production of ethyl levulinate from levulinic acid via esterification reaction in the presence of HPW/ZrO2.The catalyst was prepared using the wet impregnation method, characterized by using FTIR, BET and NH3-TPD and screened based on 20%, 40% and 60% HPW/ZrO2. The 40% HPW/ZrO2 catalyst exhibited the highest catalytic performance during the parameter screening stage which included catalyst loading (0.25‒1.25g) and volume ratio of levulinic acid to ethanol (1:4 – 1:8). The highest ethyl levulinate yield of 99% corresponded to a catalyst loading of 0.5 g and volume ratio of levulinic acid to ethanol of 1:5 with reaction conditions at 150 °C for 3 hours. -

Pdf 462.69 K
Journal of Oil, Gas and Petrochemical Technology Vol. 5, No. 1 , pp. 63 - 75 6 3 The Investigation of Purity Improvement for the Production of Methyl Propionate in Different Types of Batch Distillation Systems Dhia Y. Aqar * 1 , Hassan H. Al Alak 2 , N. Rahmanian 3 , I.M. Mujtaba 3 1 Ministry of Oil, South Refineries Company, Basra, Iraq 2 Department of Materials Engineering, Faculty of Engineering, University of Kufa, Najaf, Iraq 3 Faculty of Engineering and Informatics, University of Bradford, Bradford, UK ARTICLE INFO ABSTRACT Received: January 06, 2018 Methyl propionate, also known as methyl propanoate, Accepted: September 08, 2018 is a clear colourless liquid with a characteristic odour (fruity smel l and taste). In this study , the formation of methyl propionate through the esterification o Keywords : propionic acid and methanol was inves tigated in the Methyl propionate reactive distillation system using a conventional (CBD), CBD single feed (SF - SBD), and double feed semi - batch SF - SBD distillatio n (DF - SBD) columns for the first time . The Double feed semi - batch distillation performances measure o f these distillation systems (DF - SBD) were evaluated in terms of min imum batch time for a given separation task. The optimization results clearly * Corresponding author: show ed that the DF - SBD system is a more attractive E - mail: : [email protected] operation in terms of reaction conversion, and maximum achievable purity as compared to the SF SBD, and CBD processes. 64 Dhia Y. Aqar et al. 1. Introduction Methyl propionate is a very important component which has useful applications in a variety of areas in the chemical industry such as solvents for cellulose nitrate, lacquers, plasticizers, chemical intermediates, fragrances, flavors, a raw material in organic synthesis for the production of varnishes, paints, and other chemical compound s such as methyl methacrylate [1] . -

Unit 15 Monocarboxylic and Sulphonjc Acids
UNIT 15 MONOCARBOXYLIC AND SULPHONJC ACIDS Structure Introduction Objectives Carboxylic Acids Preparation of Monocarboxylic Acids physical Properties of ~onocarbox~licAcids Spectral Properties of Carboxylic Acids Reactions of Carboxylic Acids Sulphonic Acids Preparation of Benzenesulphonic acid Reactions of Benzenesulphonic acid Industrial Uses of Carboxylic and Sulphonic Acids Laboratory Detection of Carboxylic and Sulphonic Acids Summary Terminal Questions Answers 15.1 INTRODUCTION 0 I Carboxylic acids are the compounds which contain the carboxy (-COH) functional 0 I1 group and can be represented either as RCOH or as RCOOH. The carboxylic acids not only form an important class of organic compounds but are also the parent compounds of a large group of compounds called the functional derivatives of carboxylic acids which can be further classified as acid halides, acid anhydrides, acid .amides and esters. These classes of compounds \kill be discussed in Unit 17. Carboxylic acids also play an important role in various biological processes. In Unit 16, you will study about some such acids ..Besides carboxylic acids, there is another important class of organic acids, called sulphonic adds. The sulphonic acids are the compounds which contain a S03H group, called the sulphonic acid soup. Sulphonic acids are organic acids related to sulphuric acid. Sulphonic acids and carboxylic acids are closely related in their chemistry. Therefore, in this unit, we will first study the chemistry of carboxylic acids and then that of the sulphonic acids. Objectives -
Chemical Specificity in Short-Chain Fatty Acids and Their Analogues in Increasing Osmotic Fragility in Rat Erythrocytes in Vitro
Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic fragility in rat erythrocytes in Title vitro. Author(s) Mineo, Hitoshi; Hara, Hiroshi Biochimica et Biophysica Acta (BBA) - Biomembranes, 1768(6), 1448-1453 Citation https://doi.org/10.1016/j.bbamem.2007.02.008 Issue Date 2007-06 Doc URL http://hdl.handle.net/2115/28208 Type article (author version) File Information BBA1768-6.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP 1 1 Chemical specificity in short-chain fatty acids and their analogues in increasing osmotic 2 fragility in rat erythrocytes in vitro 3 4 Hitoshi Mineo, Hiroshi Hara* 5 6 Division of Applied Bioscience, Graduate School of Agriculture, Hokkaido University, 7 Hokkaido 060-8589, Japan 8 9 10 *Corresponding author. 11 Hiroshi HARA Ph.D. 12 Division of Applied Bioscience, 13 Graduate School of Agriculture, 14 Hokkaido University, 15 Kita-9, Nishi-9, Sapporo, 16 Hokkaido 060-8589, 17 Tel.: +81-11-706-3352; 18 fax: +81-11-706-2504. 19 E-mail address: [email protected] 20 21 22 23 24 25 2 1 Abstract 2 3 We examined the role of the chemical specificity of short-chain fatty acids 4 (SCFAs) and their derivatives in increasing osmotic fragility (OF) in rat red blood cells 5 (RBCs). Except for formic acid, normal SCFAs with 2 to 8 carbons increased the OF in 6 rat RBCs with increasing number of hydrocarbons in a dose-dependent manner. 7 Replacement of the carboxylic group with sulfonic group inhibited, but did not abolish, 8 the SCFA-mediated increase in OF.