Concepts of Chemical Bonding and Molecular Geometry Part 1 David A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Geometry Is the General Shape of a Molecule, As Determined by the Relative Positions of the Atomic Nuclei
Lecture Presentation Chapter 9 Molecular Geometries and Bonding Theories © 2012 Pearson Education, Inc. Chapter Goal • Lewis structures do not show shape and size of molecules. • Develop a relationship between two dimensional Lewis structure and three dimensional molecular shapes • Develop a sense of shapes and how those shapes are governed in large measure by the kind of bonds exist between the atoms making up the molecule © 2012 Pearson Education, Inc. Molecular geometry is the general shape of a molecule, as determined by the relative positions of the atomic nuclei. Copyright © Cengage Learning. All rights reserved. 10 | 3 The valence-shell electron-pair repulsion (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence-shell electron pairs are arranged about each atom so that electron pairs are kept as far away from one another as possible, thereby minimizing electron pair repulsions. The diagram on the next slide illustrates this. Copyright © Cengage Learning. All rights reserved. 10 | 4 Two electron pairs are 180° apart (a linear arrangement). Three electron pairs are 120° apart in one plane (a trigonal planar arrangement). Four electron pairs are 109.5° apart in three dimensions (a tetrahedral arrangment). Copyright © Cengage Learning. All rights reserved. 10 | 5 Five electron pairs are arranged with three pairs in a plane 120° apart and two pairs at 90°to the plane and 180° to each other (a trigonal bipyramidal arrangement). Six electron pairs are 90° apart (an octahedral arrangement). This is illustrated on the next slide. Copyright © Cengage Learning. All rights reserved. 10 | 6 Copyright © Cengage Learning. All rights reserved. -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Sample Exercise 9.1 Using the VSEPR Model
Sample Exercise 9.1 Using the VSEPR Model – Use the VSEPR model to predict the molecular geometry of (a) O3, (b) SnCl3 . Solution Analyze: We are given the molecular formulas of a molecule and a polyatomic ion, both conforming to the general formula ABn and both having a central atom from the p block of the periodic table. Plan: To predict the molecular geometries of these species, we first draw their Lewis structures and then count the number of electron domains around the central atom. The number of electron domains gives the electron-domain geometry. We then obtain the molecular geometry from the arrangement of the domains that are due to bonds. (a) We can draw two resonance structures for O3: Because of resonance, the bonds between the central O atom and the outer O atoms are of equal length. In both resonance structures the central O atom is bonded to the two outer O atoms and has one nonbonding pair. Thus, there are three electron domains about the central O atoms. (Remember that a double bond counts as a single electron domain.) The arrangement of three electron domains is trigonal planar (Table 9.1). Two of the domains are from bonds, and one is due to a nonbonding pair. So, the molecule has a bent shape with an ideal bond angle of 120° (Table 9.2). Chemistry: The Central Science, Eleventh Edition Copyright ©2009 by Pearson Education, Inc. By Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, and Catherine J. Murphy Upper Saddle River, New Jersey 07458 With contributions from Patrick Woodward All rights reserved. -

Chapter 9 Molecular Geometry and Bonding Theories
Secon 9.1 Hybridiza)on and the Localized Electron Model Chapter 9 Molecular Geometry and Bonding Theories Secon 9.1 Hybridiza)on and the Localized Electron Model VSEPR Model § VSEPR: Valence Shell Electron-Pair Repulsion. § The structure around a given atom is determined principally by minimizing electron pair repulsions. Copyright © Cengage Learning. All rights reserved 2 Secon 9.1 Hybridiza)on and the Localized Electron Model Steps to Apply the VSEPR Model 1. Draw the Lewis structure for the molecule. 2. Count the electron pairs and arrange them in the way that minimizes repulsion (put the pairs as far apart as possible. 3. Determine the posi$ons of the atoms from the way electron pairs are shared (how electrons are shared between the central atom and surrounding atoms). 4. Determine the name of the molecular structure from posi$ons of the atoms. Copyright © Cengage Learning. All rights reserved 3 Secon 9.1 Hybridiza)on and the Localized Electron Model Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB2 2 0 linear linear Copyright © Cengage Learning. All rights reserved Secon 9.1 Hybridiza)on and the Localized Electron Model # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry trigonal trigonal AB3 3 0 planar planar Copyright © Cengage Learning. All rights reserved Secon 9.1 Hybridiza)on and the Localized Electron Model # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB4 4 0 tetrahedral tetrahedral Copyright © Cengage Learning. -

AJR Ch10 Molecular Geometry.Docx Slide 1 Chapter 10 Molecular
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious). Molecular geometry is essentially based upon five basic geometrical structures, and can be predicted by the valence shell electron pair repulsion model (VSEPR). The VSEPR Method This method deals with electron domains, which are regions in which it is most likely to find the valence electrons. This includes the: -bonding pairs (located between two atoms, bonding domain), and -nonbonding pairs or lone pairs (located principally on one atom, nonbonding domain). (Note for bonding domains, they contain all the electrons shared between two atoms – so a multiple bond is considered as one domain). The best arrangement of a given number of electron domains (charge clouds) is the one that minimizes the repulsions among the different domains. The arrangement of electron domains about the central atom of a molecule is called its electron-domain geometry (or electronic geometry). AJR Ch10 Molecular Geometry.docx Slide 1 The five basic geometrical structures are linear, trigonal planar (planar triangular), tetrahedral, trigonal bipyramid and octahedral. Linear Trigonal Planar AJR Ch10 Molecular Geometry.docx Slide 2 Tetrahedral Trigonal Bipyramid AJR Ch10 Molecular Geometry.docx Slide 3 Octahedral The number and type of electron domains control the geometry; the number and type of electron domains is obtained from the Lewis structure. There are two general situations – either the central atom (which controls the geometry) has non-bonding electrons (lone pairs), or it does not. -

Collisions Lesson Plan VSEPR Theory Time: 1 -2 Class Periods
Collisions Lesson Plan VSEPR Theory Time: 1 -2 class periods Lesson Description In this lesson, students will use Collisions to explore molecular geometry and VSEPR Theory. Key Essential Questions 1. What is the VSEPR Theory? 2. How does the number of electron domains & lone pairs of a central atom affect molecular shape? Learning Outcomes Students will be able to determine the shape of molecular compounds using VSEPR Theory. Prior Student Knowledge Expected Atoms can covalently bond together to form molecular compounds. Lesson Materials • Individual student access to Collisions on tablet, Chromebook, or computer. • Projector / display of teacher screen • Accompanying student resources (attached) Standards Alignment NGSS Alignment Science & Enginnering Practices Disciplinary Core Ideas Crosscutting Concepts • Developing and using • HS-PS-2. Construct and • Structure and Function models revise an explanation for the • Construcing explanations outcome of a simple chemical and designing solutions rection based on the outermost electron states of atoms, trends int he periodic table, and knowl- edge of the partterns of chemi- cal properties. www.playmadagames.com ©2018 PlayMada Games LLC. All rights reserved. 1 PART 1: Explore (15 minutes) This is an inquiry-driven activity where students will build molecules in the Covalent Bonding Sandbox to begin to explore VSEPR Theory and molecular geometry. Prior to starting this lesson, students should have already completed Levels 1 -7 in the Covalent Bonding Game. A student worksheet for this activity can be found on PAGE 5. Direct students to log into Collisions with their individual username and password, enter the Covalent Bonding Sandbox and follow the prompt below, Your goal is to build 3 unique molecules in the Covalent Bonding Sandbox. -

The Complete Molecular Geometry of Salicyl Aldehyde from Rotational Spectroscopy
THE COMPLETE MOLECULAR GEOMETRY OF SALICYL ALDEHYDE FROM ROTATIONAL SPECTROSCOPY O. DOROSH, E. BIALKOWSKA-JAWORSKA, Z. KISIEL, L. PSZCZOLKOWSKI, Institute of Physics, Pol- ish Academy of Sciences, Al. Lotnikow´ 32/46, 02-668 Warszawa, Poland; M. KANSKA, T. M. KRYGOWSKI, Department of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warszawa, Poland; H. MAEDER, Insti- tut fur¨ Physikalische Chemie, Christian-Albrechts-Universitat¨ zu Kiel, Olshausenstrasse 40, D-24098 Kiel, Germany. Salicyl aldehyde is a well known planar molecule containing an internal hydrogen bond. In preparing the publication of our previous report of the study of its rotational spectruma we have taken the opportunity to update the structure determination of this molecule to SE the complete re geometry. The molecule contains 15 atoms and we have used supersonic expansion FTMW spectroscopy to obtain rotational constants for a total 26 different isotopic species, including all singly substitued species relative to the parent molecule. The 13 18 SE C and O substitutions were measured in natural abundance, while deuterium substitutions were carried out synthetically. The re determination requires the calculation of vibration-rotation changes in rotational constants from an ab initio anharmonic force field, which necessitates some compromises in the level of calculation for a molecule of the size of salicyl aldehyde. For this reason we studied the five lowest vibrationally excited states, by using the combination of room-temperature mm-wave spectroscopy and waveguide Fourier transform cm-wave spectroscopy. The experimental excited state rotational constants were then used to calibrate the anharmonic force SE field calculation. The resulting re geometry is compared with other types of geometry determination possible from this data, with emphasis on the effect of the near zero principal coordinate of the important C2 atom. -

Nomenclature and Conformations of Alkanes and Cycloalkanes1 on The
Andrew Rosen Chapter 4 - Nomenclature and Conformations of Alkanes and Cycloalkanes1 4.1 - Introduction to Alkanes and Cycloalkanes - Alkanes are hydrocarbons with all carbon-carbon single bonds - Alkenes are hydrocarbons with a carbon-carbon double bond - Alkynes are hydrocarbons with a carbon-carbon triple bond - Cycloalkanes are alkanes in which all or some of the carbon atoms are arranged in a ring - Alkanes have the general formula of CnH2n+2 - Cycloalkanes with a single ring have the formula CnH2n 4.2 - Shapes of Alkanes - An unbranched chain means that each carbon atom is bonded to no more than two other carbon atoms and that there are only primary and secondary carbon atoms 4.3 - IUPAC Nomenclature of Alkanes, Alkyl Halides, and Alcohols - Endings of alkane names end in −ane, and the standard prexes apply to how many carbon atoms there are (meth-, eth-, prop-, but-, pent-, etc.) - An alkyl group has one hydrogen removed from an alkane, and the names have a sux of −yl Rules for naming branched-chain alkanes: 1) Locate the longest continuous chain of carbon atoms. This chain determines the parent (prex) name for the alkane. Always start numbering from the end of a chain 2) Number the longest chain begining with the end of the chain nearer the substitutent 3) Use the numbers obtained by application of Rule 2 to designate the location of the substitutent group 4) When two or more substitutents are present, give each substituent a number corresponding to its location on the longest chain. Name them in alphabetical order 5) When two -

Hybrid Orbitals Hybrid Orbitals
Molecular Shape and Molecular Shape and Molecular Polarity Molecular Polarity • When there is a difference in electronegativity between • In water, the molecule is not linear and the bond dipoles two atoms, then the bond between them is polar. do not cancel each other. • It is possible for a molecule to contain polar bonds, but • Therefore, water is a polar molecule. not be polar. • For example, the bond dipoles in CO2 cancel each other because CO2 is linear. Figure 9.12 Prentice Hall © 2003 Chapter 9 Prentice Hall © 2003 Chapter 9 Molecular Shape and Molecular Shape and Molecular Polarity Molecular Polarity • The overall polarity of a molecule depends on its molecular geometry. Figure 9.13 Figure 9.11 Prentice Hall © 2003 Chapter 9 Prentice Hall © 2003 Chapter 9 1 Covalent Bonding and Covalent Bonding and Orbital Overlap Orbital Overlap • How do we account for shape in terms of quantum • As two nuclei approach each other their atomic orbitals mechanics? overlap. • What are the orbitals that are involved in bonding? • As the amount of overlap increases, the energy of the • We use Valence Bond Theory: interaction decreases. • Bonds form when orbitals on atoms overlap. • At some distance the minimum energy is reached. • There are two electrons of opposite spin in the orbital overlap. • The minimum energy corresponds to the bonding distance (or bond length). • As the two atoms get closer, their nuclei begin to repel and the energy increases. Prentice Hall © 2003 Chapter 9 Prentice Hall © 2003 Chapter 9 Covalent Bonding and Covalent Bonding and Orbital Overlap Orbital Overlap • At the bonding distance, the attractive forces between nuclei and electrons just balance the repulsive forces (nucleus-nucleus, electron-electron). -

Chapter 9. Molecular Geometry and Bonding Theories
Chapter 9. Molecular Geometry and Bonding Theories Media Resources Figures and Tables in Transparency Pack: Section: Figure 9.2 Shapes of AB2 and AB3 Molecules 9.1 Molecular Shapes Figure 9.3 Shapes Allowing Maximum Distances 9.1 Molecular Shapes between Atoms in ABn Molecules Table 9.1 Electron-Domain Geometries as a 9.2 VSEPR Model Function of Number of Electron Domains Table 9.2 Electron-Domain and Molecular 9.2 VSEPR Model Geometries for Two, Three, and Four Electron Domains around a Central Atom Table 9.3 Electron-Domain and Molecular 9.2 VSEPR Model Geometries for Five and Six Electron Domains around a Central Atom Figure 9.12 Polar and Nonpolar Molecules 9.3 Molecular Shape and Molecular Polarity Containing Polar Bonds Figure 9.14 Formation of the H2 Molecule as 9.4 Covalent Bonding and Orbital Overlap Atomic Orbitals Overlap Figure 9.15 Formation of sp Hybrid Orbitals 9.5 Hybrid Orbitals Figure 9.17 Formation of sp2 Hybrid Orbitals 9.5 Hybrid Orbitals Figure 9.18 Formation of sp3 Hybrid Orbitals 9.5 Hybrid Orbitals Table 9.4 Geometric Arrangements Characteristic 9.5 Hybrid Orbitals of Hybrid Orbital Sets Figure 9.23 The Orbital Structure of Ethylene 9.6 Multiple Bonds Figure 9.24 Formation of Two π Bonds in 9.6 Multiple Bonds Acetylene, C2H2 Figure 9.26 σ and the π Bond Networks in Benzene, 9.6 Multiple Bonds C6H6 Figure 9.27 Delocalized π Bonds in Benzene 9.6 Multiple Bonds Figure 9.32 The Two Molecular Orbitals of H2, One 9.7 Molecular Orbitals a Bonding MO and One an Antibonding MO Figure 9.35 Energy-level Diagram for the Li2 -

Molecular Geometry: VSEPR Model Stand for Valence-Shell Electron-Pair Repulsion and Predicts the 3D Shape of Molecules That Are Formed in Bonding
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that occur in the overlap of hybridized orbitals. Pi (π) bonds occur when parallel, unhybridized p orbitals overlap. Double bonds contain one sigma and one pi bond; triple bonds contain one sigma and two pi bonds. π bonds are weaker than the σ bonds, but because π bonds are found with σ bonds they are stronger than a single σ bond. Pi bonds also prevent rotation about the bond. Hybridization: Blending of outer bonding orbitals Intermolecular Forces: London Dispersion - Weak intermolecular force, temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. London (dispersion) forces are the forces that cause non-polar substances to condense to liquids and to freeze into solids when the temperature is low. Because of the constant motion of the electrons, an atom or molecule can develop a temporary (instantaneous) dipole when its electrons are distributed asymmetrically. Dipole-dipole - Forces occur between two polar molecules and result from the attractive forces between the positive end of one molecule and the negative end of another. Weaker than ionic or covalent bonds and have an effect only when the molecules involved are close together. Hydrogen bonds - A type of dipole-dipole attraction. The bonds between the hydrogen atom of one molecule and the nitrogen, oxygen or fluorine of another molecule are very short and cause a stronger polar. -
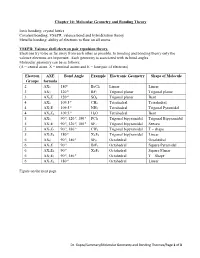
Chapter 10: Molecular Geometry and Bonding Theory
Chapter 10: Molecular Geometry and Bonding Theory Ionic bonding: crystal lattice Covalent bonding: VSEPR, valence bond and hybridization theory Metallic bonding: ability of electrons to flow on all atoms. VSEPR: Valence shell electron pair repulsion theory. Electrons try to be as far away from each other as possible. In bonding and bonding theory only the valence electrons are important. Each geometry is associated with its bond angles. Molecular geometry can be as follows: (A = central atom, X = terminal atoms and E = lone pair of electrons) Electron AXE Bond Angle Example Electronic Geometry Shape of Molecule Groups formula o 2 AX2 180 BeCl2 Linear Linear o 3 AX3 120 BF3 Trigonal planar Trigonal planar o 3 AX2E 120 SO2 Trigonal planar Bent o 4 AX4 109.5 CH4 Tetrahedral Tetrahedral o 4 AX3E 109.5 NH3 Tetrahedral Trigonal Pyramidal o 4 AX2E2 109.5 H2O Tetrahedral Bent o o o 5 AX5 90 , 120 , 180 PCl5 Trigonal bipyramidal Trigonal Bipyramidal o o o 5 AX4E 90 , 120 , 180 SF4 Trigonal bipyramidal Seesaw o o 5 AX3E2 90 , 180 CIF4 Trigonal bipyramidal T – shape o 5 AX2E3 180 XeF2 Trigonal bipyramidal Linear o o 6 AX6 90 , 180 SF6 Octahedral Octahedral o 6 AX5E 90 BrF5 Octahedral Square Pyramidal o 6 AX4E2 90 XeF4 Octahedral Square Planar o o 6 AX3E3 90 , 180 Octahedral T – Shape o 6 AX2E4 180 Octahedral Linear Figure on the next page. Dr. Gupta/Summary/Molecular Geometry and Bonding Theories/Page 1 of 3 No of Geometry 1 Lone Pair 2 Lone Pairs 3 Lone Pairs 4 Lone Pairs e- (all atoms) groups 2 Linear AX2 X A X 3 Trigonal Bent Planar X AX2E .