Bibliography of Nickel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Pennsylvania State University Center for Critical Minerals Final
The Pennsylvania State University Center for Critical Minerals Final Report on Cobalt Production in Pennsylvania: Context and Opportunities PREPARED FOR LEONARDO TECHNOLOGIES LLC., PREPARED BY Peter L. Rozelle Feifei Shi Mohammad Rezaee Sarma V. Pisupati Disclaimer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. ii Executive Summary Cobalt is a critical mineral as defined by the U.S. Department of the Interior in response to Executive Order 13817 (2017). Cobalt metal is used in superalloys required in the hot gas path of stationary gas turbines and jet engines, as well as in samarium cobalt magnets, the domestic production capability of which was found to be essential to the National Defense under Presidential Determination 2019-20. Cobalt compounds are used in lithium-ion batteries, as are manganese compounds. The Defense Industrial Base Report, in response to Executive Order 13806 (2017), expressed concerns regarding the U.S. -

LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, and COBALT ARSENIDE and SULFIDE ORE FORMATION Nicholas Allin
Montana Tech Library Digital Commons @ Montana Tech Graduate Theses & Non-Theses Student Scholarship Spring 2019 EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION Nicholas Allin Follow this and additional works at: https://digitalcommons.mtech.edu/grad_rsch Part of the Geotechnical Engineering Commons EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION by Nicholas C. Allin A thesis submitted in partial fulfillment of the requirements for the degree of Masters in Geoscience: Geology Option Montana Technological University 2019 ii Abstract Experiments were conducted to determine the relative rates of reduction of aqueous sulfate and aqueous arsenite (As(OH)3,aq) using foils of copper, nickel, or cobalt as the reductant, at temperatures of 150ºC to 300ºC. At the highest temperature of 300°C, very limited sulfate reduction was observed with cobalt foil, but sulfate was reduced to sulfide by copper foil (precipitation of Cu2S (chalcocite)) and partly reduced by nickel foil (precipitation of NiS2 (vaesite) + NiSO4·xH2O). In the 300ºC arsenite reduction experiments, Cu3As (domeykite), Ni5As2, or CoAs (langisite) formed. In experiments where both sulfate and arsenite were present, some produced minerals were sulfarsenides, which contained both sulfide and arsenide, i.e. cobaltite (CoAsS). These experiments also produced large (~10 µm along longest axis) euhedral crystals of metal-sulfide that were either imbedded or grown upon a matrix of fine-grained metal-arsenides, or, in the case of cobalt, metal-sulfarsenide. Some experimental results did not show clear mineral formation, but instead demonstrated metal-arsenic alloying at the foil edges. -

Canadian Money Word Search Extension Activity for Earn, Spend, Save & Share, I Need It! I Want It! Or Spending Sense Presentations
Canadian Money Word Search Extension Activity for Earn, Spend, Save & Share, I Need It! I Want It! or Spending Sense Presentations Grade Level: Grades 1-3 Learning Objective: This extension activity, along with the Earn, Spend, Save & Share, I Need It! I Want It! or Spending Sense presentations should help students: • learn common money terms • develop their visual ability for recognizing words related to money Materials Needed: • Canadian Money Word Search & pencil (1 per student) Preparation: 1. Review the meanings of the money words found in the worksheet: dollar: a unit of money equal to 100 cents bill: paper money which is also called notes. Bills represent larger amounts of money than coins. Canadian bills are produced at the bank of Canada located in Ottawa, Ontario nickel: a coin worth five cents loonie: a coin worth 100 cents/one dollar twenty: a number equal in dollar value to a green Canadian bill ($20 bill). Twenty dollars can also be represented by several combinations of bills and coins of smaller value coin: round pieces of metal used as money. Canada has five coins (nickel, dime, quarter, loonie, toonie). Coins represent smaller values of money than bills. Canadian coins are produced at the royal Canadian mint located in Winnipeg, Manitoba dime: a coin worth ten cents toonie: a coin worth 200 cents/ two dollars fifty: a number equal in dollar value to a red Canadian bill ($50 bill). Fifty dollars can also be represented by a several combinations of bills and coins of smaller value quarter: a coin worth twenty-five cents ten: a number equal in dollar value to a purple Canadian bill ($10 bill). -

Overview of the Nickel Market in Latin America and the Caribbean
INSG SECRETARIAT BRIEFING PAPER April 2021 – No.35 Overview of the Nickel market in Latin America and the Caribbean Ricardo Ferreira, Director of Market Research and Statistics Francisco Pinto, Manager of Statistical Analysis 1. Introduction At the suggestion of the Group’s Brazilian delegation, it was agreed by members that a report, based on INSG Insight No. 26, published in November 2015 entitled “An overview of the nickel industry in Latin America”, be prepared by the secretariat. This would include an update of the operations that resumed production (e.g. Falcondo in the Dominican Republic), and also discuss how the emerging battery market might influence nickel usage in the region as well as the possibility of nickel scrap usage. This detailed and comprehensive Insight report, the 35th in the series of INSG Insight briefing reports, provides members with the results of that research work. In Latin America and the Caribbean, the nickel producing countries are Brazil, Colombia, Cuba, Dominican Republic, Guatemala and Venezuela. The Dominican Republic stopped nickel mining and smelting in October 2013 but resumed production at the beginning of 2016. Venezuela has not produced since mid-2015. All of these countries mine nickel and process it further to produce intermediate or primary nickel – mainly ferronickel. Most of the mined ore is processed within each country, and then exported to overseas markets, but Brazil and Guatemala also export nickel ore. In terms of usage only Brazil and Mexico are relevant regarding the global nickel market. The first section of this report briefly describes existing nickel deposits in the region. -

Rebel Attacks on Nickel Ore Mines in the Surigao Del Norte Region
Mindanao, Philippines – rebel attacks on nickel ore mines in the Surigao del Norte region The attacks On 3 October 2011, three mining firms located in and around Claver town in the Surigao del Norte region of Mindanao, Philippines were attacked by the rebel group known as the New People’s Army (NPA). It was reported that the attacks were prompted by a refusal of the mining firms to pay a "revolutionary tax" demanded by the NPA. The affected mining firms are the Taganito Mining Corporation (TMC), Taganito HPAL Nickel Corporation and Platinum Group Metals Corporation (PGMC)1. According to news reports and Gard’s local correspondent in the Philippines, some 300 NPA rebels wearing police/military uniforms staged coordinated strikes on the three mining facilities. The rebels attacked TMC’s compound first, burning heavy equipment, service vehicles, barges and buildings. Other rebel groups simultaneously attacked the nearby PGCM and Taganito HPAL compounds. Unconfirmed reports indicate that, unfortunately, casualties were also experienced during the attacks. All foreign vessels anchored near Taganito at the time of the attacks left the anchorage safely. The current situation The Philippine Government has stated that the situation is now contained and has deployed additional troops in the area to improve security and pursue the NPA rebels. However, according to Gard’s local correspondent in the Philippines, threats of further attacks made by the NPA in a press release are believed by many to be real. According to the latest press releases (Reuters on 5 October), Nickel Asia Corporation has resumed operations at its biggest nickel mine TMC and expects to ship ore in the next three weeks. -
Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254)
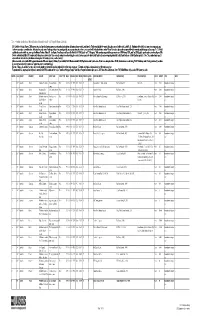
Table1.—Attribute data for the map "Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254). [The United States Geological Survey (USGS) surveys international mineral industries to generate statistics on the global production, distribution, and resources of industrial minerals. This directory highlights the economically significant mineral facilities of Asia and the Pacific. Distribution of these facilities is shown on the accompanying map. Each record represents one commodity and one facility type for a single location. Facility types include mines, oil and gas fields, and processing plants such as refineries, smelters, and mills. Facility identification numbers (“Position”) are ordered alphabetically by country, followed by commodity, and then by capacity (descending). The “Year” field establishes the year for which the data were reported in Minerals Yearbook, Volume III – Area Reports: Mineral Industries of Asia and the Pacific. In the “DMS Latitiude” and “DMS Longitude” fields, coordinates are provided in degree-minute-second (DMS) format; “DD Latitude” and “DD Longitude” provide coordinates in decimal degrees (DD). Data were converted from DMS to DD. Coordinates reflect the most precise data available. Where necessary, coordinates are estimated using the nearest city or other administrative district.“Status” indicates the most recent operating status of the facility. Closed facilities are excluded from this report. In the “Notes” field, combined annual capacity represents the total of more facilities, plus additional -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -

Nickeline Nias C 2001-2005 Mineral Data Publishing, Version 1
Nickeline NiAs c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6/m 2/m 2/m. Commonly in granular aggregates, reniform masses with radial structure, and reticulated and arborescent growths. Rarely as distorted, horizontally striated, {1011} terminated crystals, to 1.5 cm. Twinning: On {1011} producing fourlings; possibly on {3141}. Physical Properties: Fracture: Conchoidal. Tenacity: Brittle. Hardness = 5–5.5 VHN = n.d. D(meas.) = 7.784 D(calc.) = 7.834 Optical Properties: Opaque. Color: Pale copper-red, tarnishes gray to blackish; white with strong yellowish pink hue in reflected light. Streak: Pale brownish black. Luster: Metallic. Pleochroism: Strong; whitish, yellow-pink to pale brownish pink. Anisotropism: Very strong, pale greenish yellow to slate-gray in air. R1–R2: (400) 39.2–45.4, (420) 38.0–44.2, (440) 36.8–43.5, (460) 36.2–43.2, (480) 37.2–44.3, (500) 39.6–46.4, (520) 42.3–48.6, (540) 45.3–50.7, (560) 48.2–52.8, (580) 51.0–54.8, (600) 53.7–56.7, (620) 55.9–58.4, (640) 57.8–59.9, (660) 59.4–61.3, (680) 61.0–62.5, (700) 62.2–63.6 Cell Data: Space Group: P 63/mmc. a = 3.621(1) c = 5.042(1) Z = 2 X-ray Powder Pattern: Unknown locality. 2.66 (100), 1.961 (90), 1.811 (80), 1.071 (40), 1.328 (30), 1.033 (30), 0.821 (30) Chemistry: (1) (2) Ni 43.2 43.93 Co 0.4 Fe 0.2 As 55.9 56.07 Sb 0.1 S 0.1 Total 99.9 100.00 (1) J´achymov, Czech Republic; by electron microprobe, corresponds to (Ni0.98Co0.01 Fe0.01)Σ=1.00As1.00. -

Handbook of Iron Meteorites, Volume 2 (Bitburg
326 Bishop Canyon - Bitburg fibrous and brecciated, and 1-100 p wide veins through it ' l ' j are filled with a glass in which silicate fragments and a little ) ·. / troilite are embedded.lt is surprising to note how the shock . e·· f . has been able to create such heavy local transformation; 10 mm away nothing unusual is observed. The local damage resembles in several respects the structural changes associated with spot welding. Examination under the electron microprobe suggests that the unknown silicate is ~' almost pure Si02 , possibly tridymite. Bishop Canyon is chemically and structurally a typical ~ phosphorus-poor, fine octahedrite of groupiVA, . l .. resembling in particular Gibe on. It is, however, unusual in .0 its silicate inclusions, the shock damage and low hardness. ,, .• ~: :t • ·. • cr ··. @ Specimen in the U.S. National Museum in Washington: .. ~ - ,·r -. f '-' f 226 g slice (no. 770, 9.5 x 4.5 x 0.9 em) II . 0 t' / •. ' ~~ ~~ {) r ...~ · -· · ...· ~· -/~... Figure 352. Bitburg. Detail of Figure 350. The dendritic metal was Bitburg, Rhineland, Germany at high temperature austenite but transformed upon cooling to unequilibrated a • Etched. Scale bar 50 f.l. 49°58'N, 6°32'E 2 A mass said to have weighed 1.5 tons was smelted in a furnace before 1805. It was recognized as a meteorite (Chladni 1819: 353), but virtually nothing was saved of the undamaged material (Hey 1966: 57). The smelted material, e.g., no. 1881, 1534 of 546 gin .. \ . • I : '/ 'I \ ' . ~\.. // .·· Figure 350. Bit burg (Copenhagen no. 1886, 493). A rectangular bar cut from melted material of the Bitburg meteorite. -

ENG-JAN14 Web.Pdf
COINS FROM THE ROYAL CANADIAN MINT 2014 | NUMBER 1 PRESERVED foreVER UNFORGETTABLE WITH WORLD- MOMENTS RENOWNED COINS. AT BOUTIQUES AND MINT.CA startING JanuarY 7 153rd BATTalION IN TraINIng. SOurCE: Canada. DEPT. OF NATIONAL DEFenCE / LIbrarY and ARCHIVES Canada / PA-022759 THE POWER OF A WAR-TIME EMBRACE. When Britain declared war on Germany on August 4, 1914, its entire Empire was drawn into the conflict, including Canada. Across the Dominion, men flocked to recruiting stations. Within two months, Canada’s pre-war militia that included a standing army of 3,110 men had grown to 33,000. Many were recent British immigrants or native-born Canadians of British origin, but among them were also more than 1,000 French Canadians, many First Nations as well as many others from diverse ethnic backgrounds. Five hundred soldiers from the British colonies of Newfoundland and Labrador also joined the ranks, while some 2,500 women stepped forward to serve as nurses. Train stations across Canada became the stage for tearful goodbyes and lingering embraces. The First World War was a true coming of age for the young nation, and the hope, fear, courage and deep sacrifice Canadians felt 100 years ago remain as poignant and inspiring today. Designed by Canadian artist Bonnie Ross, this coin depicts a couple’s emotional farewell as the first wave of volunteers boards for camp. Time stands still for this couple as they savour one last embrace before his departure. It is a poignant reminder of the sacrifices made by those who answered the call of duty, and their loved ones who remained on the home front. -

The Tawallah Valley Meteorite. General Description. the Microstructure.Records of the Australian Museum 21(1): 1–8, Plates I–Ii
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Hodge-Smith, T., and A. B. Edwards, 1941. The Tawallah Valley meteorite. General description. The Microstructure.Records of the Australian Museum 21(1): 1–8, plates i–ii. [4 July 1941]. doi:10.3853/j.0067-1975.21.1941.518 ISSN 0067-1975 Published by the Australian Museum, Sydney nature culture discover Australian Museum science is freely accessible online at http://publications.australianmuseum.net.au 6 College Street, Sydney NSW 2010, Australia THE TAW ALLAH VALLEY METEORITE. General Description. By T. HODGE-SMI'l'H, The Australian Museum. The Microstructure. By A. B. EDWARDS, Ph.D., D.I.C.,* Research Officer, Mineragraphy Branch, Council for Scientific and Industrial Research. (Plates i-ii and Figures 1-2.) General Description. Little information is available about the finding of this meteorite. Mr. Heathcock, Constable-in-Charge of the Borroloola Police Station, Northern Territory, informed me in April, 1939, that it had been in the Police Station for eighteen months or more. It was found by Mr. Condon, presumably some time in 1937. The weight of the iron as received was 75·75 kg. (167 lb.). A small piece had been cut off, but its weight probably did not exceed 200 grammes. The main mass weighing 39·35 kg. (86i lb.) is in the collection of the Geological Survey, Department of the Interior, Canberra. A portion weighing 30·16 kg. (66~ lb.) and five pieces together weighing 1·67 kg. are in the collection of the Australian Museum, and a slice weighing 453 grammes is in the Museum of the Geology Department, the University of Melbourne. -

Nickel and Nickel Compounds Were Considered by Previous !AC Working Groups, in 1972, 1975, 1979, 1982 and 1987 (IARC, 1973, 1976, 1979, 1982, 1987)
NICKEL AND NieKEL eOMPOUNDS Nickel and nickel compounds were considered by previous !AC Working Groups, in 1972, 1975, 1979, 1982 and 1987 (IARC, 1973, 1976, 1979, 1982, 1987). Since that time, new data have become available, and these are inc1uded in the pres- ent monograph and have been taken into consideration in the evaluation. 1. ehemical and Physical Data The list of nickel alloys and compounds given in Table 1 is not exhaustive, nor does it necessarily reflect the commercial importance of the various nickel-con tain- ing substances, but it is indicative of the range of nickel alloys and compounds avail- able, including some compounds that are important commercially and those that have been tested in biological systems. A number of intermediary compounds occur in refineries which cannot be characterized and are not listed. 1.1 Synonyms, trade names and molecular formulae of nickel and selected nickel-containing compounds Table 1. Synonyms (Chemical Abstracts Service names are given in bold), trade names and atomic or molecular formulae or compositions of nickel, nickel alloys and selected nickel compounds Chemical Chem. Abstr. SYDoDyms and trade Dames Formula Dame Seiv. Reg. Oxda- Numbera tion stateb Metallc nickel and nickel alloys Nickel 7440-02-0 c.I. 77775; NI; Ni 233; Ni 270; Nickel 270; Ni o (8049-31-8; Nickel element; NP 2 17375-04-1; 39303-46-3; 53527-81-4; 112084-17-0) -- 257 - NICKEL AND NICKEL COMPOUNDS 259 Table i (contd) Chemical Chem. Abstr. Synonym and trade names Formula name Seiv. Reg. Ox- Number4 dation stateb