Thermodynamics of Ideal Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On Entropy, Information, and Conservation of Information
entropy Article On Entropy, Information, and Conservation of Information Yunus A. Çengel Department of Mechanical Engineering, University of Nevada, Reno, NV 89557, USA; [email protected] Abstract: The term entropy is used in different meanings in different contexts, sometimes in contradic- tory ways, resulting in misunderstandings and confusion. The root cause of the problem is the close resemblance of the defining mathematical expressions of entropy in statistical thermodynamics and information in the communications field, also called entropy, differing only by a constant factor with the unit ‘J/K’ in thermodynamics and ‘bits’ in the information theory. The thermodynamic property entropy is closely associated with the physical quantities of thermal energy and temperature, while the entropy used in the communications field is a mathematical abstraction based on probabilities of messages. The terms information and entropy are often used interchangeably in several branches of sciences. This practice gives rise to the phrase conservation of entropy in the sense of conservation of information, which is in contradiction to the fundamental increase of entropy principle in thermody- namics as an expression of the second law. The aim of this paper is to clarify matters and eliminate confusion by putting things into their rightful places within their domains. The notion of conservation of information is also put into a proper perspective. Keywords: entropy; information; conservation of information; creation of information; destruction of information; Boltzmann relation Citation: Çengel, Y.A. On Entropy, 1. Introduction Information, and Conservation of One needs to be cautious when dealing with information since it is defined differently Information. Entropy 2021, 23, 779. -

ENERGY, ENTROPY, and INFORMATION Jean Thoma June
ENERGY, ENTROPY, AND INFORMATION Jean Thoma June 1977 Research Memoranda are interim reports on research being conducted by the International Institute for Applied Systems Analysis, and as such receive only limited scientific review. Views or opinions contained herein do not necessarily represent those of the Institute or of the National Member Organizations supporting the Institute. PREFACE This Research Memorandum contains the work done during the stay of Professor Dr.Sc. Jean Thoma, Zug, Switzerland, at IIASA in November 1976. It is based on extensive discussions with Professor HAfele and other members of the Energy Program. Al- though the content of this report is not yet very uniform because of the different starting points on the subject under consideration, its publication is considered a necessary step in fostering the related discussion at IIASA evolving around th.e problem of energy demand. ABSTRACT Thermodynamical considerations of energy and entropy are being pursued in order to arrive at a general starting point for relating entropy, negentropy, and information. Thus one hopes to ultimately arrive at a common denominator for quanti- ties of a more general nature, including economic parameters. The report closes with the description of various heating appli- cation.~and related efficiencies. Such considerations are important in order to understand in greater depth the nature and composition of energy demand. This may be highlighted by the observation that it is, of course, not the energy that is consumed or demanded for but the informa- tion that goes along with it. TABLE 'OF 'CONTENTS Introduction ..................................... 1 2 . Various Aspects of Entropy ........................2 2.1 i he no me no logical Entropy ........................ -

Practice Problems from Chapter 1-3 Problem 1 One Mole of a Monatomic Ideal Gas Goes Through a Quasistatic Three-Stage Cycle (1-2, 2-3, 3-1) Shown in V 3 the Figure
Practice Problems from Chapter 1-3 Problem 1 One mole of a monatomic ideal gas goes through a quasistatic three-stage cycle (1-2, 2-3, 3-1) shown in V 3 the Figure. T1 and T2 are given. V 2 2 (a) (10) Calculate the work done by the gas. Is it positive or negative? V 1 1 (b) (20) Using two methods (Sackur-Tetrode eq. and dQ/T), calculate the entropy change for each stage and ∆ for the whole cycle, Stotal. Did you get the expected ∆ result for Stotal? Explain. T1 T2 T (c) (5) What is the heat capacity (in units R) for each stage? Problem 1 (cont.) ∝ → (a) 1 – 2 V T P = const (isobaric process) δW 12=P ( V 2−V 1 )=R (T 2−T 1)>0 V = const (isochoric process) 2 – 3 δW 23 =0 V 1 V 1 dV V 1 T1 3 – 1 T = const (isothermal process) δW 31=∫ PdV =R T1 ∫ =R T 1 ln =R T1 ln ¿ 0 V V V 2 T 2 V 2 2 T1 T 2 T 2 δW total=δW 12+δW 31=R (T 2−T 1)+R T 1 ln =R T 1 −1−ln >0 T 2 [ T 1 T 1 ] Problem 1 (cont.) Sackur-Tetrode equation: V (b) 3 V 3 U S ( U ,V ,N )=R ln + R ln +kB ln f ( N ,m ) V2 2 N 2 N V f 3 T f V f 3 T f ΔS =R ln + R ln =R ln + ln V V 2 T V 2 T 1 1 i i ( i i ) 1 – 2 V ∝ T → P = const (isobaric process) T1 T2 T 5 T 2 ΔS12 = R ln 2 T 1 T T V = const (isochoric process) 3 1 3 2 2 – 3 ΔS 23 = R ln =− R ln 2 T 2 2 T 1 V 1 T 2 3 – 1 T = const (isothermal process) ΔS 31 =R ln =−R ln V 2 T 1 5 T 2 T 2 3 T 2 as it should be for a quasistatic cyclic process ΔS = R ln −R ln − R ln =0 cycle 2 T T 2 T (quasistatic – reversible), 1 1 1 because S is a state function. -
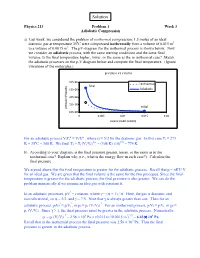
3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

Chapter 3. Second and Third Law of Thermodynamics
Chapter 3. Second and third law of thermodynamics Important Concepts Review Entropy; Gibbs Free Energy • Entropy (S) – definitions Law of Corresponding States (ch 1 notes) • Entropy changes in reversible and Reduced pressure, temperatures, volumes irreversible processes • Entropy of mixing of ideal gases • 2nd law of thermodynamics • 3rd law of thermodynamics Math • Free energy Numerical integration by computer • Maxwell relations (Trapezoidal integration • Dependence of free energy on P, V, T https://en.wikipedia.org/wiki/Trapezoidal_rule) • Thermodynamic functions of mixtures Properties of partial differential equations • Partial molar quantities and chemical Rules for inequalities potential Major Concept Review • Adiabats vs. isotherms p1V1 p2V2 • Sign convention for work and heat w done on c=C /R vm system, q supplied to system : + p1V1 p2V2 =Cp/CV w done by system, q removed from system : c c V1T1 V2T2 - • Joule-Thomson expansion (DH=0); • State variables depend on final & initial state; not Joule-Thomson coefficient, inversion path. temperature • Reversible change occurs in series of equilibrium V states T TT V P p • Adiabatic q = 0; Isothermal DT = 0 H CP • Equations of state for enthalpy, H and internal • Formation reaction; enthalpies of energy, U reaction, Hess’s Law; other changes D rxn H iD f Hi i T D rxn H Drxn Href DrxnCpdT Tref • Calorimetry Spontaneous and Nonspontaneous Changes First Law: when one form of energy is converted to another, the total energy in universe is conserved. • Does not give any other restriction on a process • But many processes have a natural direction Examples • gas expands into a vacuum; not the reverse • can burn paper; can't unburn paper • heat never flows spontaneously from cold to hot These changes are called nonspontaneous changes. -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -

Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho a Dissertation
Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems By Tony Ho A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Engineering – Mechanical Engineering in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair Professor Van P. Carey Professor Per F. Peterson Spring 2012 Abstract Advanced Organic Vapor Cycles for Improving Thermal Conversion Efficiency in Renewable Energy Systems by Tony Ho Doctor of Philosophy in Mechanical Engineering University of California, Berkeley Professor Ralph Greif, Co-Chair Professor Samuel S. Mao, Co-Chair The Organic Flash Cycle (OFC) is proposed as a vapor power cycle that could potentially increase power generation and improve the utilization efficiency of renewable energy and waste heat recovery systems. A brief review of current advanced vapor power cycles including the Organic Rankine Cycle (ORC), the zeotropic Rankine cycle, the Kalina cycle, the transcritical cycle, and the trilateral flash cycle is presented. The premise and motivation for the OFC concept is that essentially by improving temperature matching to the energy reservoir stream during heat addition to the power cycle, less irreversibilities are generated and more power can be produced from a given finite thermal energy reservoir. In this study, modern equations of state explicit in Helmholtz energy such as the BACKONE equations, multi-parameter Span- Wagner equations, and the equations compiled in NIST REFPROP 8.0 were used to accurately determine thermodynamic property data for the working fluids considered. Though these equations of state tend to be significantly more complex than cubic equations both in form and computational schemes, modern Helmholtz equations provide much higher accuracy in the high pressure regions, liquid regions, and two-phase regions and also can be extended to accurately describe complex polar fluids. -

HEAT and TEMPERATURE Heat Is a Type of ENERGY. When Absorbed
HEAT AND TEMPERATURE Heat is a type of ENERGY. When absorbed by a substance, heat causes inter-particle bonds to weaken and break which leads to a change of state (solid to liquid for example). Heat causing a phase change is NOT sufficient to cause an increase in temperature. Heat also causes an increase of kinetic energy (motion, friction) of the particles in a substance. This WILL cause an increase in TEMPERATURE. Temperature is NOT energy, only a measure of KINETIC ENERGY The reason why there is no change in temperature at a phase change is because the substance is using the heat only to change the way the particles interact (“stick together”). There is no increase in the particle motion and hence no rise in temperature. THERMAL ENERGY is one type of INTERNAL ENERGY possessed by an object. It is the KINETIC ENERGY component of the object’s internal energy. When thermal energy is transferred from a hot to a cold body, the term HEAT is used to describe the transferred energy. The hot body will decrease in temperature and hence in thermal energy. The cold body will increase in temperature and hence in thermal energy. Temperature Scales: The K scale is the absolute temperature scale. The lowest K temperature, 0 K, is absolute zero, the temperature at which an object possesses no thermal energy. The Celsius scale is based upon the melting point and boiling point of water at 1 atm pressure (0, 100o C) K = oC + 273.13 UNITS OF HEAT ENERGY The unit of heat energy we will use in this lesson is called the JOULE (J). -

Thermodynamics Formulation of Economics Burin Gumjudpai A
Thermodynamics Formulation of Economics 306706052 NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 Burin Gumjudpai A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics) School of Development Economics National Institute of Development Administration 2019 Thermodynamics Formulation of Economics Burin Gumjudpai School of Development Economics Major Advisor (Associate Professor Yuthana Sethapramote, Ph.D.) The Examining Committee Approved This Thesis Submitted in Partial 306706052 Fulfillment of the Requirements for the Degree of Master of Economics (Financial Economics). Committee Chairperson NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 (Assistant Professor Pongsak Luangaram, Ph.D.) Committee (Assistant Professor Athakrit Thepmongkol, Ph.D.) Committee (Associate Professor Yuthana Sethapramote, Ph.D.) Dean (Associate Professor Amornrat Apinunmahakul, Ph.D.) ______/______/______ ABST RACT ABSTRACT Title of Thesis Thermodynamics Formulation of Economics Author Burin Gumjudpai Degree Master of Economics (Financial Economics) Year 2019 306706052 We consider a group of information-symmetric consumers with one type of commodity in an efficient market. The commodity is fixed asset and is non-disposable. We are interested in seeing if there is some connection of thermodynamics formulation NIDA E-THESIS 5710313001 thesis / recv: 09012563 15:07:41 seq: 16 to microeconomics. We follow Carathéodory approach which requires empirical existence of equation of state (EoS) and coordinates before performing maximization of variables. In investigating EoS of various system for constructing the economics EoS, unexpectedly new insights of thermodynamics are discovered. With definition of truly endogenous function, criteria rules and diagrams are proposed in identifying the status of EoS for an empirical equation. -

Nuclear Astrophysics
Nuclear Astrophysics Lecture 3 Thurs. Nov. 3, 2011 Prof. Shawn Bishop, Office 2013, Ex. 12437 www.nucastro.ph.tum.de 1 Summary of Results Thus Far 2 Alternative expressions for Pressures is the number of atoms of atomic species with atomic number “z” in the volume V Mass density of each species is just: where are the atomic mass of species “z” and Avogadro’s number, respectively Mass fraction, in volume V, of species “z” is just And clearly, Collect the algebra to write And so we have for : If species “z” can be ionized, the number of particles can be where is the number of free particles produced by species “z” (nucleus + free electrons). If fully ionized, and 3 The mean molecular weight is defined by the quantity: We can write it out as: is the average of for atomic species Z > 2 For atomic species heavier than helium, average atomic weight is and if fully ionized, Fully ionized gas: Same game can be played for electrons: 4 Temp. vs Density Plane Relativistic - Relativistic Non g cm-3 5 Thermodynamics of the Gas 1st Law of Thermodynamics: Thermal energy of the system (heat) Total energy of the system Assume that , then Substitute into dQ: Heat capacity at constant volume: Heat capacity at constant pressure: We finally have: 6 For an ideal gas: Therefore, And, So, Let’s go back to first law, now, for ideal gas: using For an isentropic change in the gas, dQ = 0 This leads to, after integration of the above with dQ = 0, and 7 First Law for isentropic changes: Take differentials of but Use Finally Because g is constant, we can integrate -

Heat Energy a Science A–Z Physical Series Word Count: 1,324 Heat Energy
Heat Energy A Science A–Z Physical Series Word Count: 1,324 Heat Energy Written by Felicia Brown Visit www.sciencea-z.com www.sciencea-z.com KEY ELEMENTS USED IN THIS BOOK The Big Idea: One of the most important types of energy on Earth is heat energy. A great deal of heat energy comes from the Sun’s light Heat Energy hitting Earth. Other sources include geothermal energy, friction, and even living things. Heat energy is the driving force behind everything we do. This energy gives us the ability to run, dance, sing, and play. We also use heat energy to warm our homes, cook our food, power our vehicles, and create electricity. Key words: cold, conduction, conductor, convection, energy, evaporate, fire, friction, fuel, gas, geothermal heat, geyser, heat energy, hot, insulation, insulator, lightning, liquid, matter, particles, radiate, radiant energy, solid, Sun, temperature, thermometer, transfer, volcano Key comprehension skill: Cause and effect Other suitable comprehension skills: Compare and contrast; classify information; main idea and details; identify facts; elements of a genre; interpret graphs, charts, and diagram Key reading strategy: Connect to prior knowledge Other suitable reading strategies: Ask and answer questions; summarize; visualize; using a table of contents and headings; using a glossary and bold terms Photo Credits: Front cover: © iStockphoto.com/Julien Grondin; back cover, page 5: © iStockphoto.com/ Arpad Benedek; title page, page 20 (top): © iStockphoto.com/Anna Ziska; pages 3, 9, 20 (bottom): © Jupiterimages Corporation;